Methods and compositions for diagnostic and therapeutic targeting of cox-2
A technology of COX-2 and diagnostic agents, applied in the field of derivatives of non-steroidal anti-inflammatory drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0453] Synthesis of representative therapeutic analogs
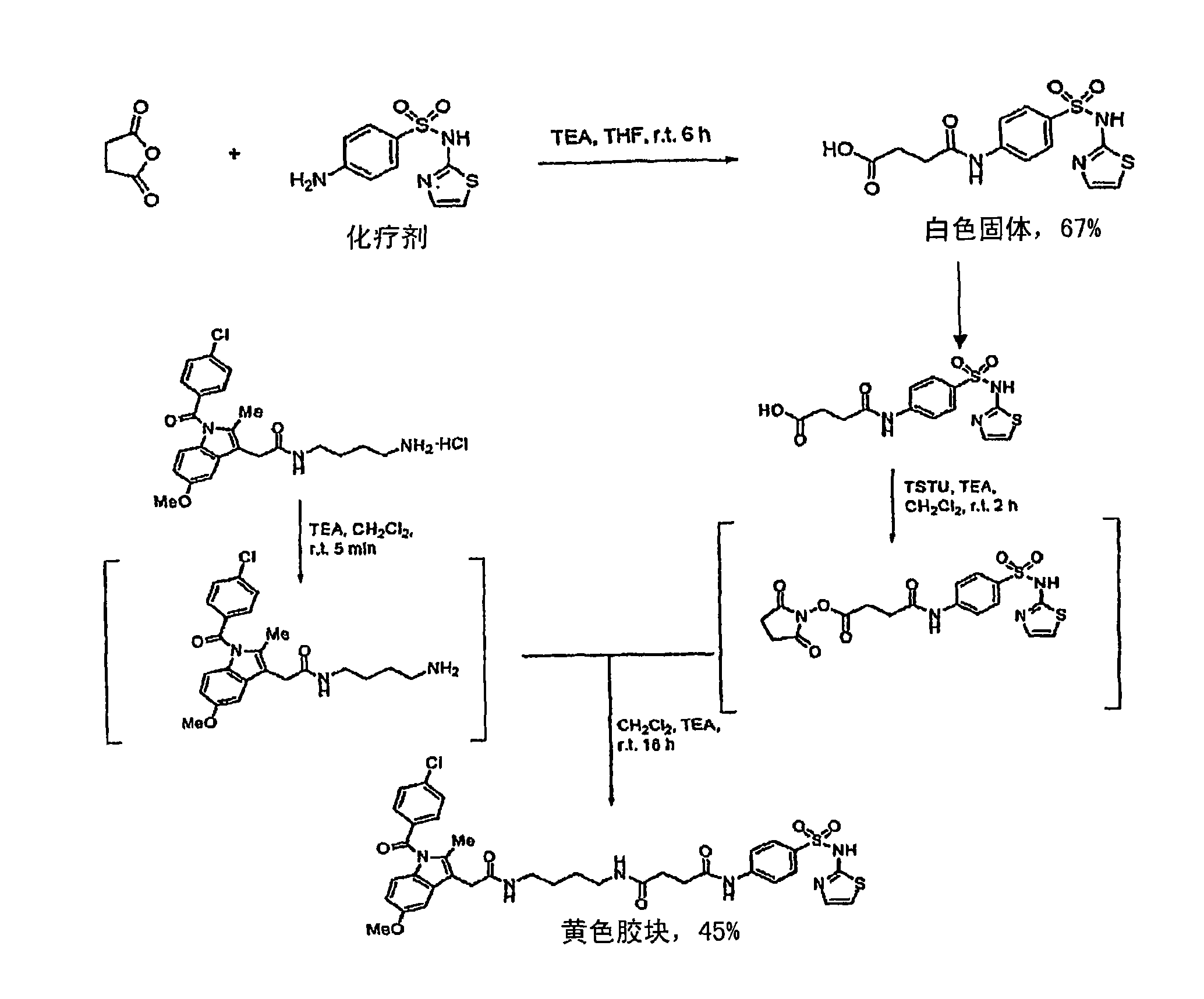
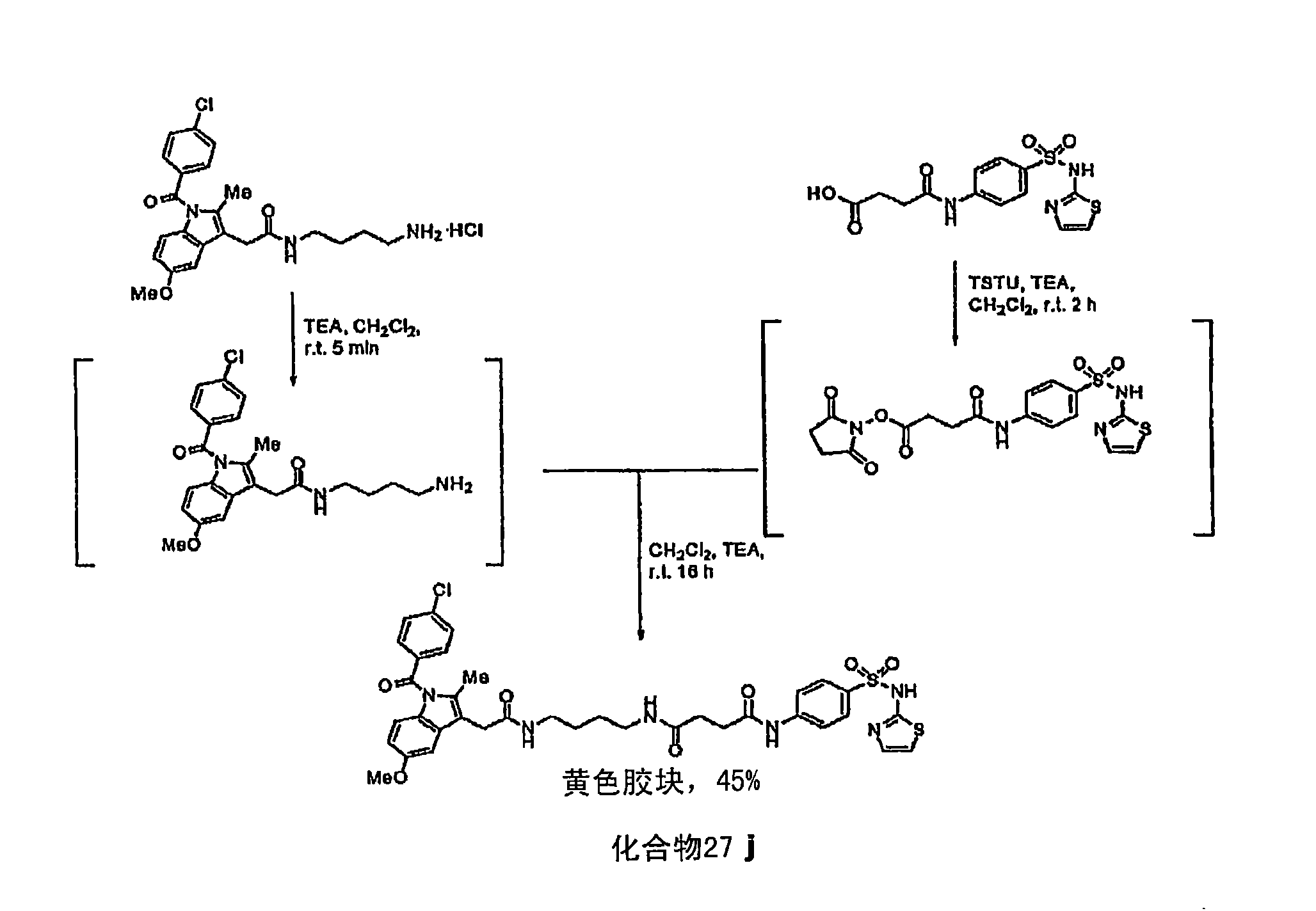
[0454] Compound 27j: Indo-sulfathiazole analog :pass figure 1 Compound 27j was synthesized by the method described in . Briefly, compound 27j was synthesized by first complexing succinic anhydride and sulfathiazole in tetrahydrofuran (THF) at room temperature in the presence of triethylamine (TEA) for 6 hours to prepare a white solid. succinylsulfathiazole (67% yield). Subsequently, at room temperature and in the presence of TEA, succinylsulfathiazole was mixed with O-(N-succinimidyl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TSTU) in dichloromethane 2 hours to prepare the corresponding succinimide ester, then at room temperature and in the presence of TEA, the succinimide ester and 1-(4-aminobutyl)-2-{1-(4-chlorobenzene Formyl)-5-methoxy-2-methyl-1H-indol-3-yl}acetamide was reacted in dichloromethane for 16 hours to prepare compound 27j as a yellow gum (45% yield). Compound 27j was characterized by NMR, two...
Embodiment 2
[0482] Synthesis of Representative Diagnostic Analogs
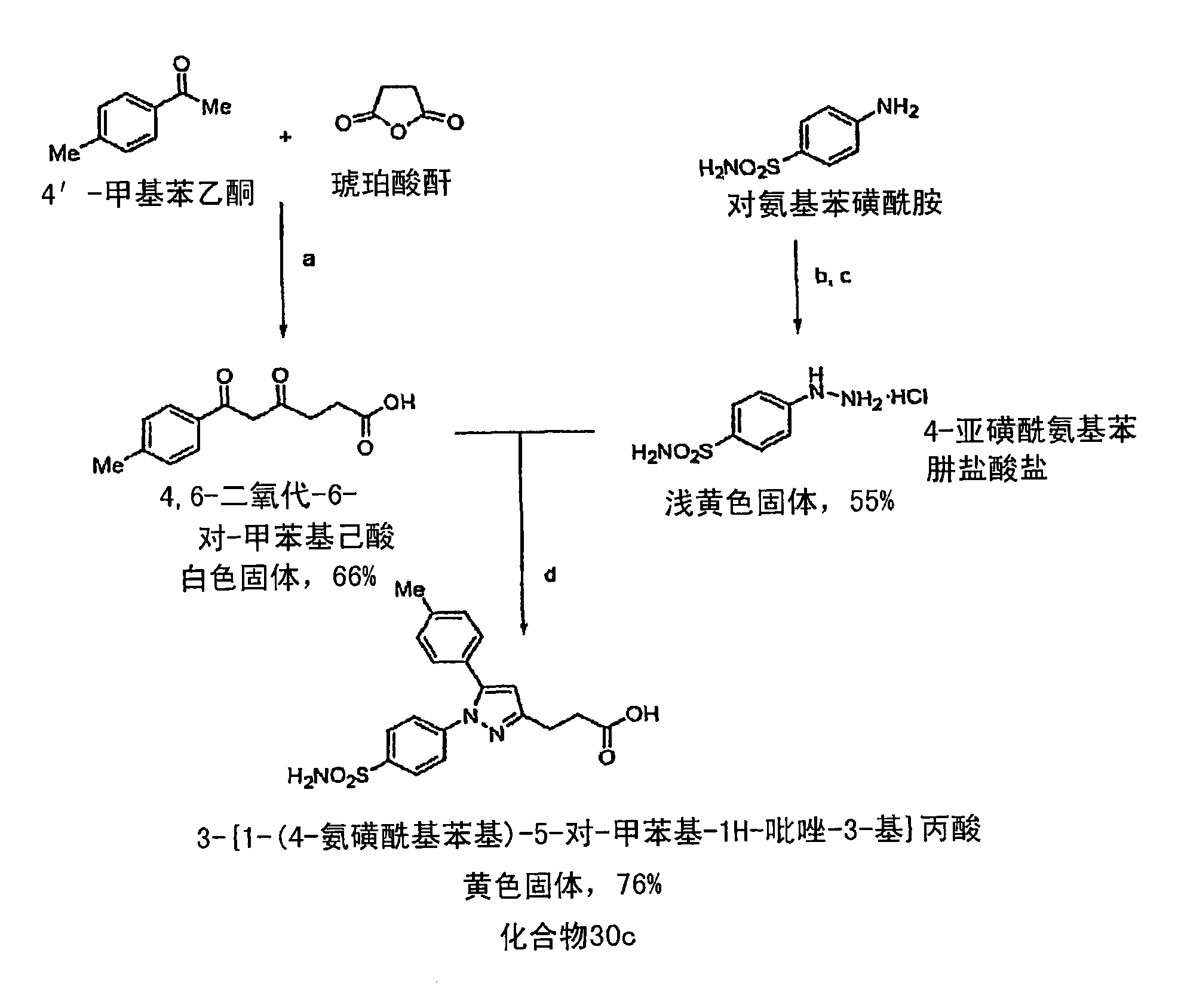
[0483] Compound 27z: Fluorescent Indo-dansyl analog :pass Figure 4 Compound 27z was synthesized by the method described in . Briefly, at room temperature and 1-ethyl-3-(3'-dimethylaminopropyl)carbodiimide hydrochloride (EDCI), 1-hydroxybenzotriazole (HOBt), dimethyl Complexation of indomethacin with N-BOC butanediamine in the presence of diaminopyridine (DMAP), N,N'-diisopropylethylamine (DIPEA) and N,N-dimethylformamide (DMF) 16 hours. A yellow solid was obtained corresponding to a percent yield of 72%. This solid was then dissolved in dichloromethane and treated with HCl(g) at room temperature for 2 hours to produce N-(4-aminobutyl)-2-[1-(4-chlorobenzyl) as a brown solid Acyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetamide hydrochloride (98% yield), N-(4 -Aminobutyl)-2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetamide hydrochloride and triethylamine ( TEA) was reacted in dichloromethane for 5 minute...
Embodiment 3
[0530] Determination of IC using purified enzyme 50
[0531] Cyclooxygenase activity of sheep COX-1 (44 nM) or human COX-2 (130 nM) was analyzed by TLC. A 200 μL reaction mixture was composed of hematin-reconstituted protein in 100 mM Tris-HCl (pH 8.0), 500 μM phenol and [1- 14 C]-arachidonic acid (50 μM, about 55-57 mCi / mmol; PerkinElmer Life And Analytical Sciences, Inc., Wellesley, Massachusetts, United States of America). For the analysis of inhibition over time, the heme-reconstituted protein was preincubated for 17 minutes at room temperature, and then preincubated in DMSO for 3 minutes at 37°C using various concentrations of the composition of the present disclosure, followed by Add [1- 14 C]-Arachidonic acid (50 [mu]M) for 30 sec. in Et 2 O / CH 3 The reaction was terminated by solvent extraction in OH / 1M citrate (pH 4.0, 30:4:1). The phases were separated by centrifugation at 2000 g for 5 minutes and the organic phase was spotted onto 20x20 cm TLC plates (Liese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com