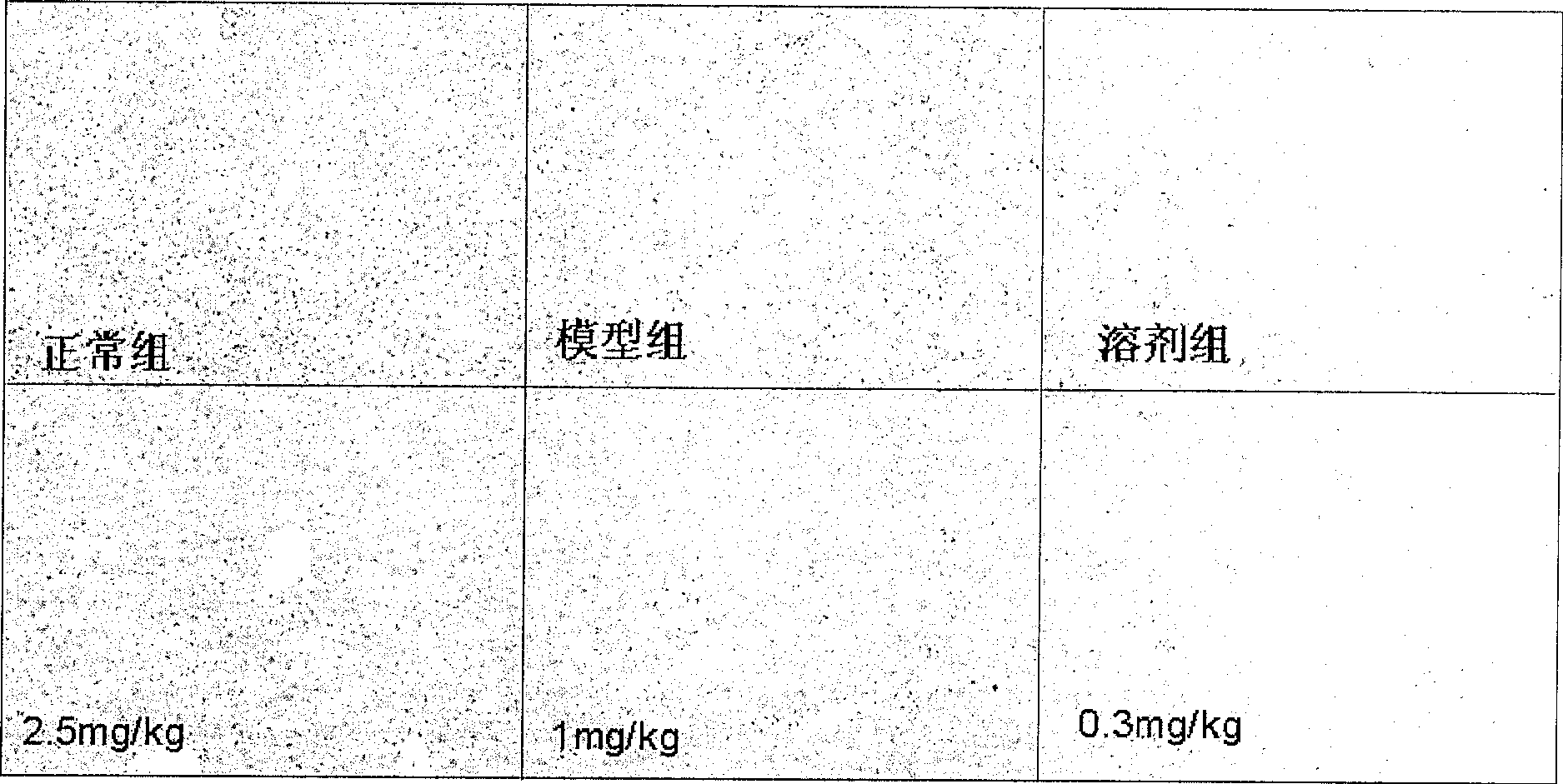
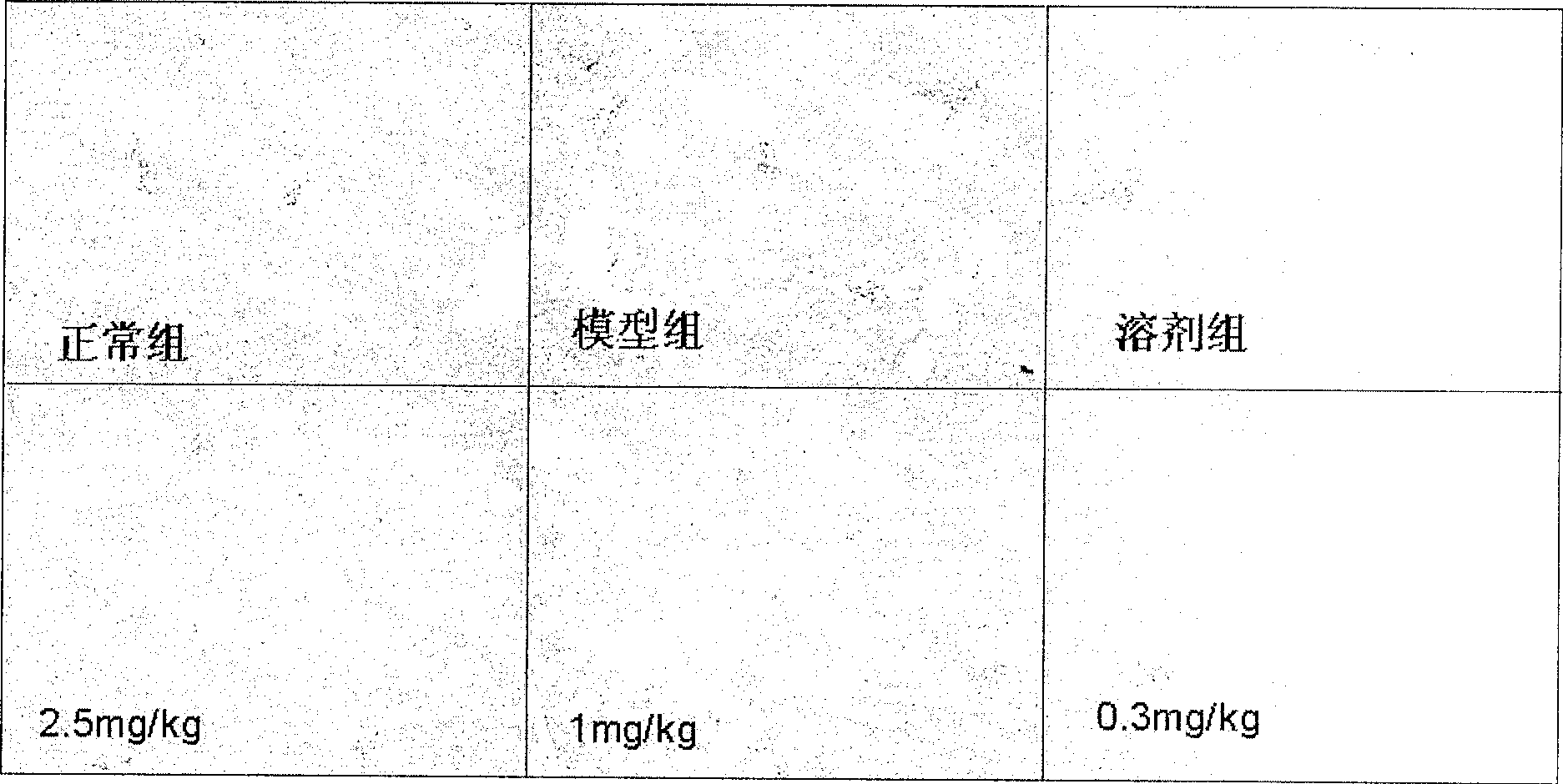
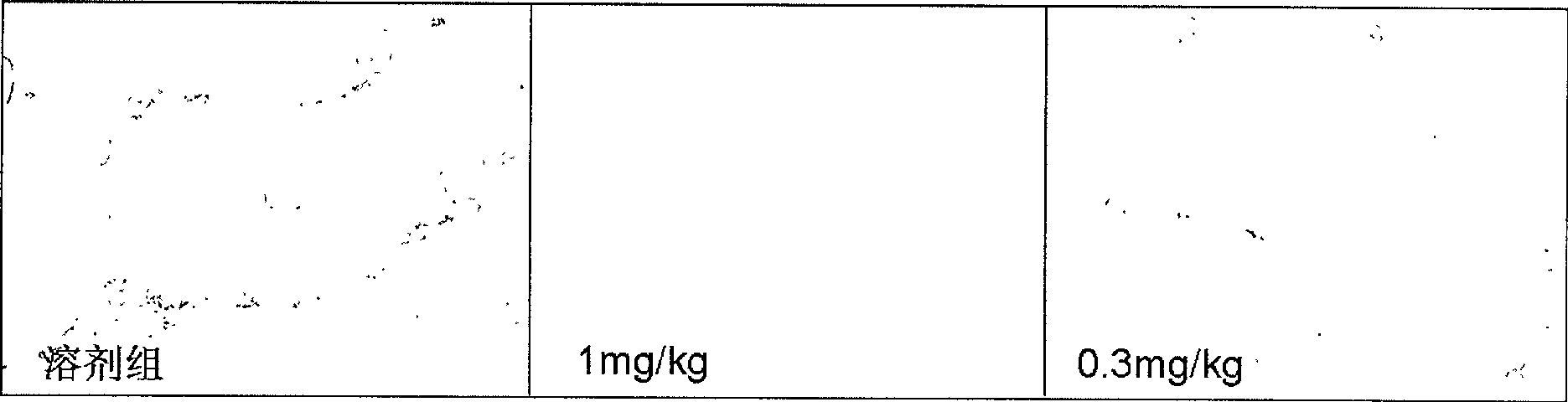Application of dammarane triterpenes derivative and drug composition containing dammarane triterpenes derivative
A technology of dammarane triterpenoids and derivatives, applied in the field of medicinal chemistry, can solve problems such as Gynostemma pentaphyllum that cannot be further explained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1000 grams of Gynostemma pentaphyllum was refluxed with 5L petroleum ether and extracted 3 times for 3 hours each time, and the obtained dregs were extracted 3 times with methanol refluxed for 3 hours each time. Concentrate the methanol extract under reduced pressure to obtain 70 g of Gynostemma pentaphyllum extractum, mix the samples with Gynostemma pentaphyllum extractum and silica gel, carry out gradient column chromatography elution with chloroform:methanol 100:10-100:50, and 100:20 elution fraction Concentrate under reduced pressure to obtain 15g, then eluted with MeOH with Sephadex LH-20 to remove flavonoids, then eluted with water: acetone 9:1-2:1 gradient through MCI resin column, combined 9:1 eluted fractions 1g was recovered under reduced pressure, and then MeOH:H 2 O=6:4 via RP-C 18 320 mg of pure compound 1 was obtained by elution, with a yield of 0.46%. Combined MCI resin 8:1 eluted fractions were recovered under reduced pressure to obtain 1.2g, and then ...
Embodiment 2
[0037] Compound 1 (1 g) obtained in Example 1 was hydrolyzed with 2N hydrochloric acid for 2 hours, distributed with equal volumes of ethyl acetate and water, after three extractions, the organic phases were combined, washed successively with saturated aqueous sodium carbonate solution and saturated brine , and then dried over anhydrous sodium sulfate overnight. Concentrated under reduced pressure to obtain 490 mg of crude compound 5, which was eluted through a silica gel column with petroleum ether: acetone = 9:1 to obtain 250 mg of pure compound 5 with a yield of 48.3%.
Embodiment 3
[0039] Compound 2 (1 g) obtained in Example 1 was hydrolyzed with 2N hydrochloric acid for 2 hours, distributed with equal volumes of ethyl acetate and water, after three extractions, the organic phases were combined, and washed successively with saturated aqueous sodium carbonate and saturated brine , and then dried over anhydrous sodium sulfate overnight. Concentrated under reduced pressure to obtain 451 mg of crude compound 6, which was eluted through a silica gel column with petroleum ether: acetone = 8:1 to obtain 210 mg of pure compound 6 with a yield of 40.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com