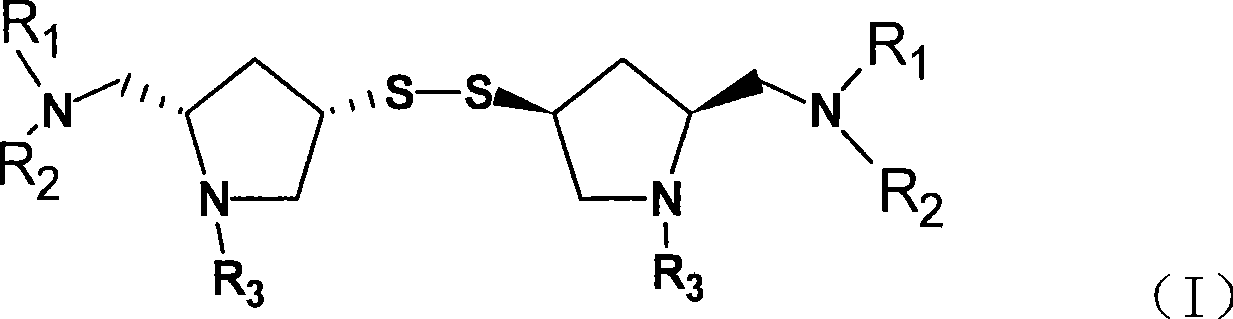
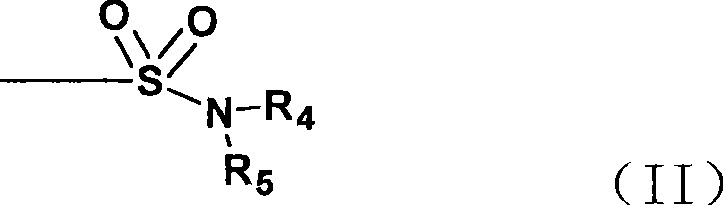
Method for preparing Doripenem and important intermediate thereof
A technology of intermediates and compounds, applied in the field of preparation of doripenem, which can solve the problems of difficult product purification, difficult removal of aluminum ions, waste, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Step 1, preparation of bis[(2S, 4S)-1-tert-butoxycarbonyl-2-hydroxymethyl-pyrrolidin-4 base]-disulfide:
[0021] In a 250ml three-necked flask, add (2S, 4S)-1-tert-butoxycarbonyl-2-hydroxymethyl-4-mercaptopyrrolidine crude product (CN92111069 Example 4, product of step 7) (6.93g, 30mmol) and Methanol (50ml) was stirred and dissolved, the temperature was lowered to below 0°C in an ice-salt bath, ferric chloride methanol solution (0.02g / 20ml) was added, and oxygen was passed through at 45°C for 10-15 hours for oxidation. The solid was precipitated by suction filtration, and was filtered and dried to obtain the product (4.8 g, yield 69%).
[0022] Step 2, preparation of bis[(2S,4S)-1-tert-butoxycarbonyl-2-(sulfonamidoamino)methyl-pyrrolidin-4 base]-disulfide:
[0023] In a dry 500ml three-necked flask, add the product from the previous step (4.6g, 0.01mol) and tetrahydrofuran (460ml), then add diethyl azodicarboxylate (DEAD) (3.31g), N-tert-butyl hydroxythio Amide (3g), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com