Modulators of chemokine receptor activity, crystalline forms and process
A crystalline form, compound technology, applied in the direction of organic active ingredients, non-central analgesics, anti-inflammatory agents, etc., can solve problems such as reducing the tendency of clinical drug-drug interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0132] The present disclosure also provides novel methods of making compounds of Formula I:
[0133]
[0134] Including N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-2-oxo-3-(6-(trifluoromethyl)quinazoline-4- (amino)pyrrolidin-1-yl)cyclohexyl)acetamide, or a pharmaceutically acceptable salt thereof.
[0135] In the first embodiment, the present disclosure provides a novel process for preparing the compound of formula IV, the process comprising:
[0136] The amino acid derivative of structural formula III or its salt is coupled with formula II cyclohexanone or its salt (refer to the preparation in WO2005021500) to obtain the compound of structural formula IV or its salt with substituted amide side chains
[0137]
[0138] in:
[0139] R a with R b independently for C 1-6 alkoxy;
[0140] or R a with R b and the carbon to which they are both attached to form a carbonyl, thiocarbonyl, cyclic acetal or cyclic thioacetal, wherein the cyclic acetal or cyclic thioacetal is s...
Embodiment 1
[0449] N-((1R, 2S, 5R)-5-(tert-butylamino)-2-((S)-2-oxo-3-(6-(trifluoromethyl)quinazolin-4-yl Amino)pyrrolidin-1-yl)cyclohexyl)acetamide
[0450]
[0451] Example 1, step 1: making (1R, 2S, 5R)-2-benzyloxycarbonylamino-7-oxo-6-aza-bicyclo[3.2.1]octane-6-carboxylic acid tert-butyl ester ( 89.6 g, 0.24 mol, see: P.H Carter et al., PCT application WO 2005 / 021500) was dissolved in ethyl acetate (1.5 liters), and the resulting solution was distilled with saturated NaHCO 3 (2 x 0.45 L) and saturated NaCl (1 x 0.45 L). The solution was dehydrated to dryness (Na 2 SO 4 ), then filtered directly into a 3-neck 3-liter round bottom flask. The solution was scrubbed with direct nitrogen injection and then charged with 10% Pd / C (13.65 g) under a nitrogen atmosphere. The flask was evacuated and backfilled with hydrogen; this was repeated two more times. Hydrogen was bubbled through the solution for 30 min, then the reactants were heated at 1 atm H 2 Stirring was continued for 18 ho...
Embodiment 2
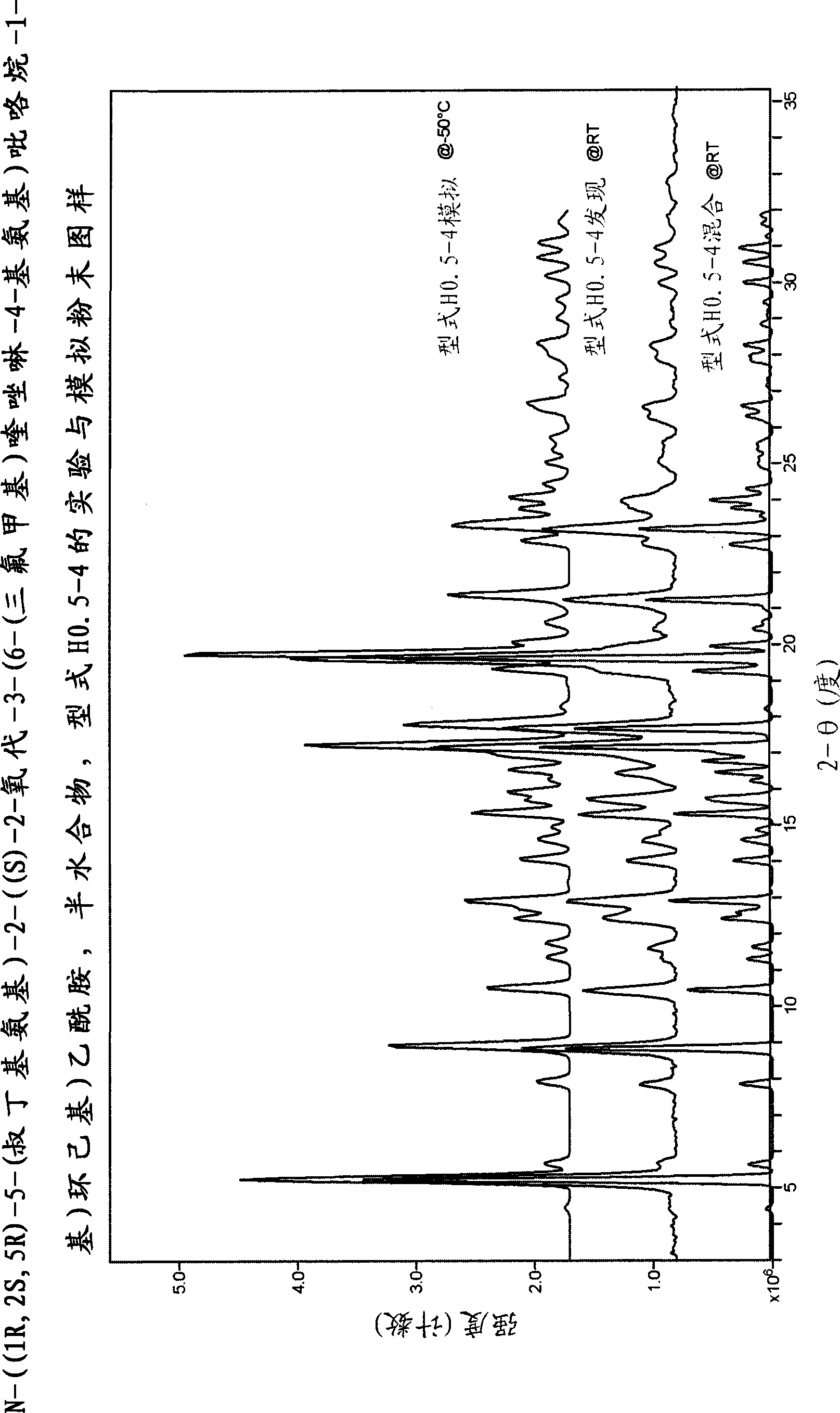
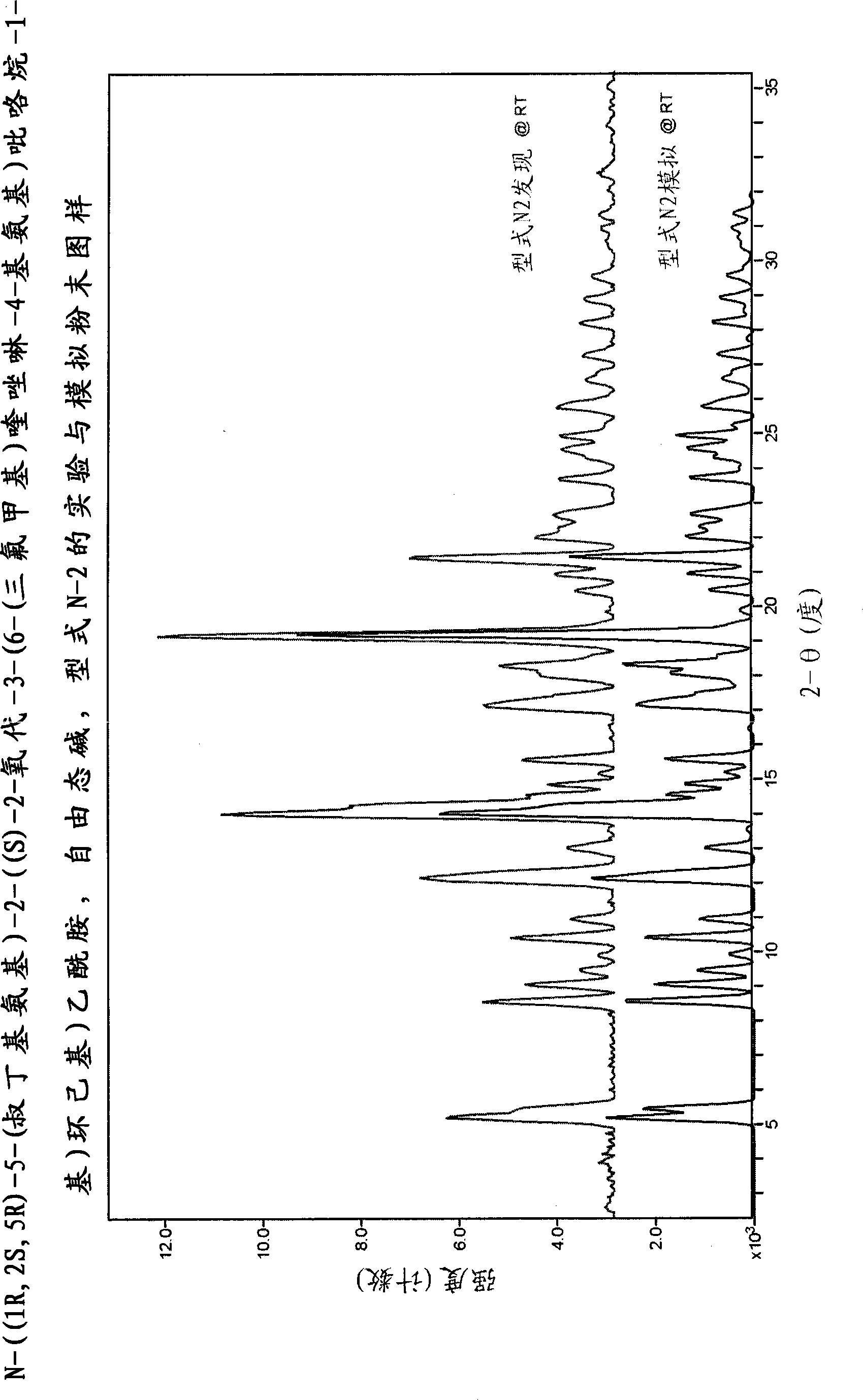
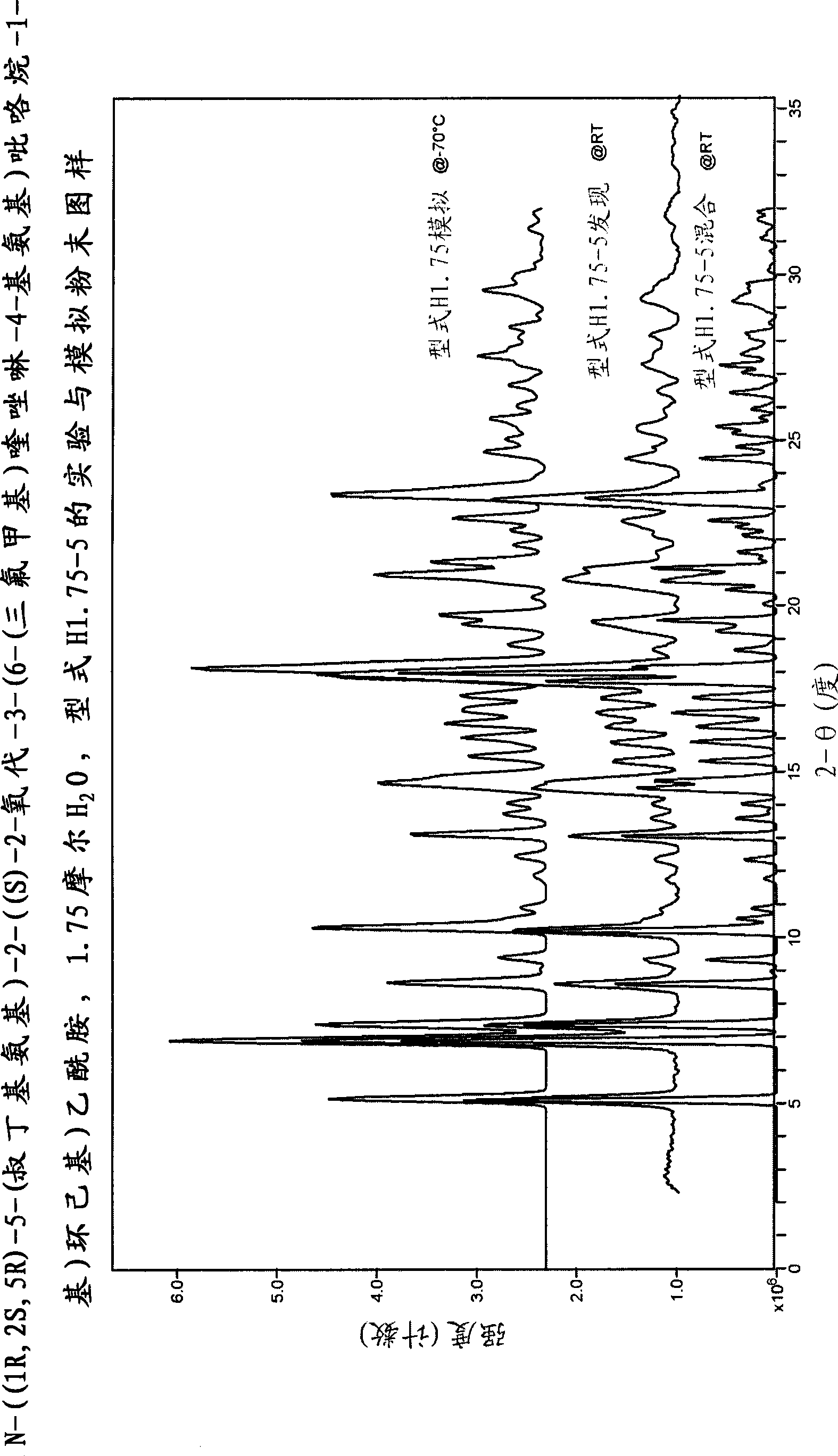
[0497] N-((1R, 2S, 5R)-5-(tert-butylamino)-2-((S)-2-oxo-3-(6-(trifluoromethyl)quinazolin-4-yl Crystalline form of amino)pyrrolidin-1-yl)cyclohexyl)acetamide
[0498] N-((1R, 2S, 5R)-5-(tert-butylamino)-2-((S)-2-oxo-3-(6-(trifluoromethyl)quinazolin-4-yl Various crystalline forms of amino)pyrrolidin-1-yl)cyclohexyl)acetamide free base were prepared and characterized according to the following methods.
[0499] Procedures for characterization of each type
[0500] single crystal data
[0501] Data were collected on a Bruker-Nonius (BRUKER AXS Corporation, 5465 East Cheryl Parkway Madison, WI 53711 USA) CAD4 serial diffractometer. The unit cell parameters were obtained by least squares analysis with an experimental diffractometer setup of 25 high-angle reflections. Intensities are measured using Cu Kα radiation (λ=1.5418 ), at a constant temperature, measured with the θ-2θ variable scanning technique, and only corrected for the Lorentz-polarizability factor. Background coun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com