Application of N-(thiofuran-2) pyrazolo (1, 5-a) pyridine-3-formanides compounds for preparing antineoplastic
A technology of carboxamides and compounds is applied in the research field of anti-tumor drugs, which can solve the problems of few reports and restricting anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
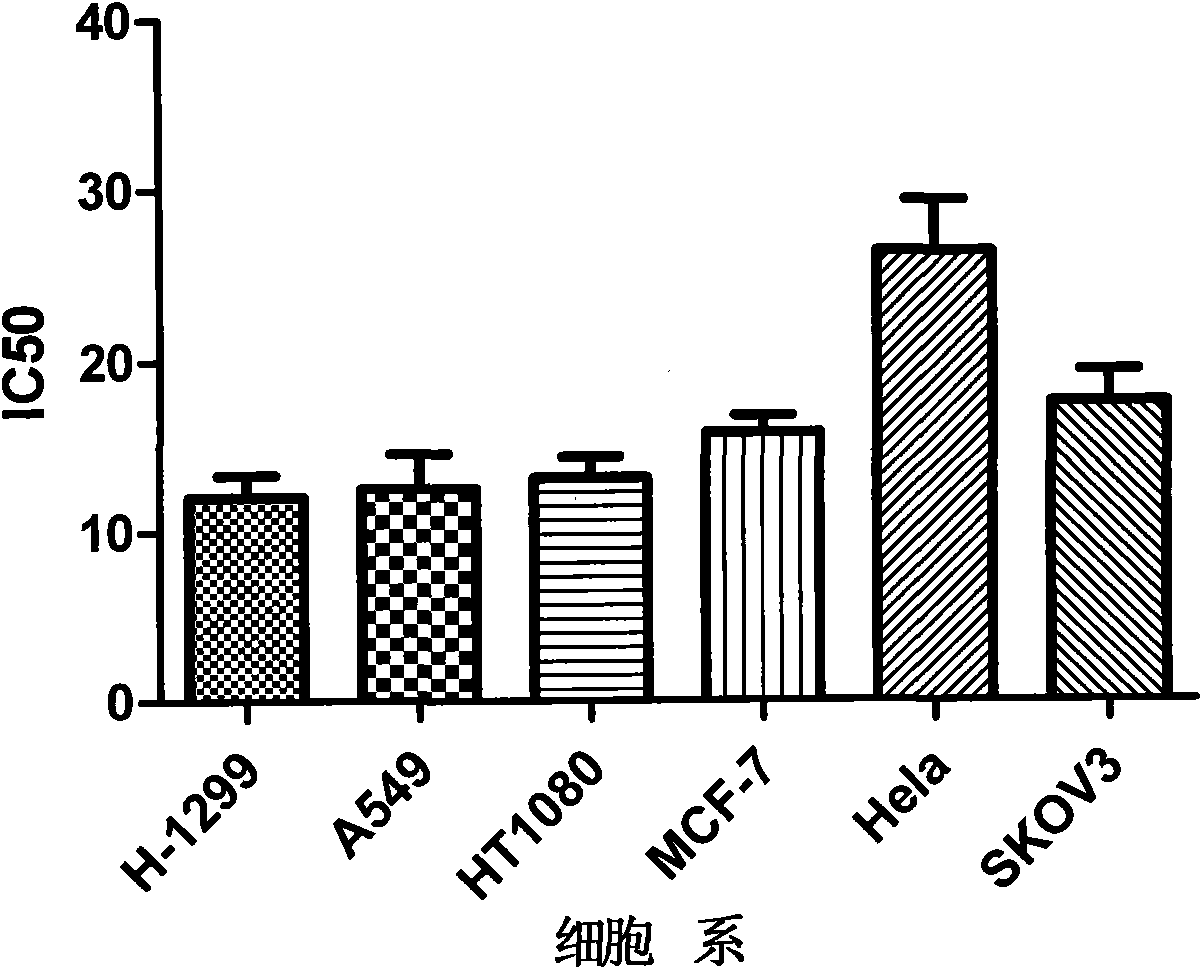
[0040] Embodiment 1: MMT detects the inhibitory effect of compound on tumor cells
[0041] The proliferation inhibitory activity of each compound on tumor cell lines was determined by conventional MTT method. H1299 cells in the logarithmic growth phase were used for preliminary activity experiments, and the cell concentration was adjusted to 1×10 with RPMI1640 culture medium containing 10% serum. 5 / ml, inoculated in a 96-well culture plate, 100 μl per well, and cultured for 24 hours at 37°C and 5% CO2. Add drugs in groups, set three parallel wells for each concentration, add different concentrations of drugs to the treatment group, and add equal volume of PBS to the negative control group, after 48 hours of incubation, add 20 μl of 5 mg / ml MTT to each well, and continue to incubate at 37°C for 4 hours Discard the supernatant, add 150 μl DMSO to each well, shake until the precipitate is completely dissolved, and measure the optical density (OD) value at 570 nm on a microplate...
Embodiment 2
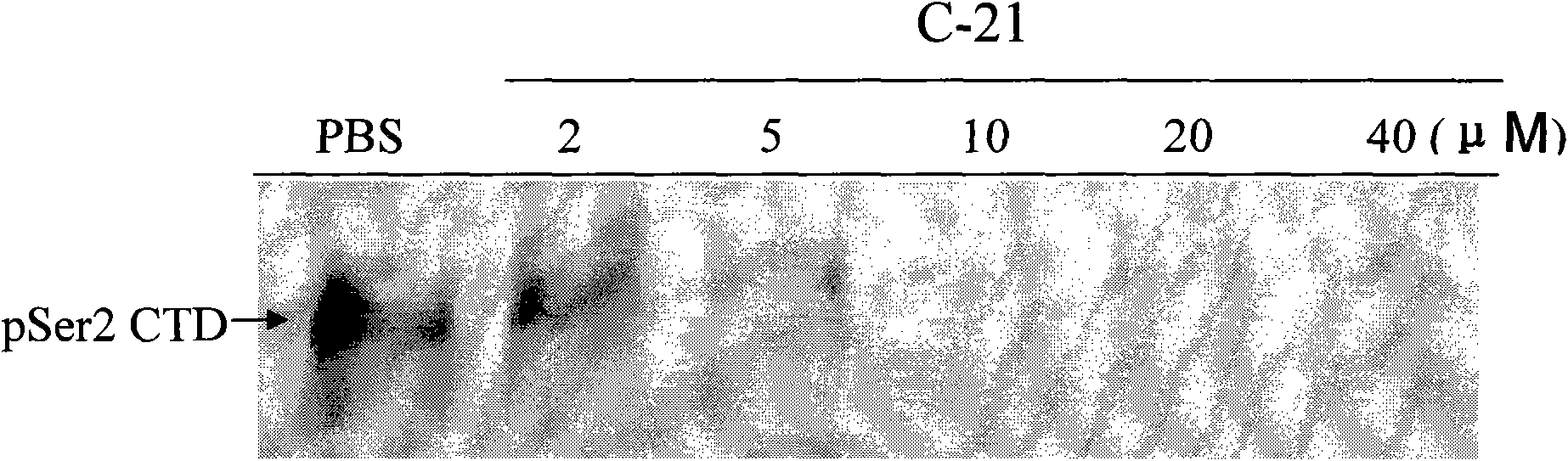
[0050] Example 2: Inhibition experiment of C-21 on the kinase action of CDK9
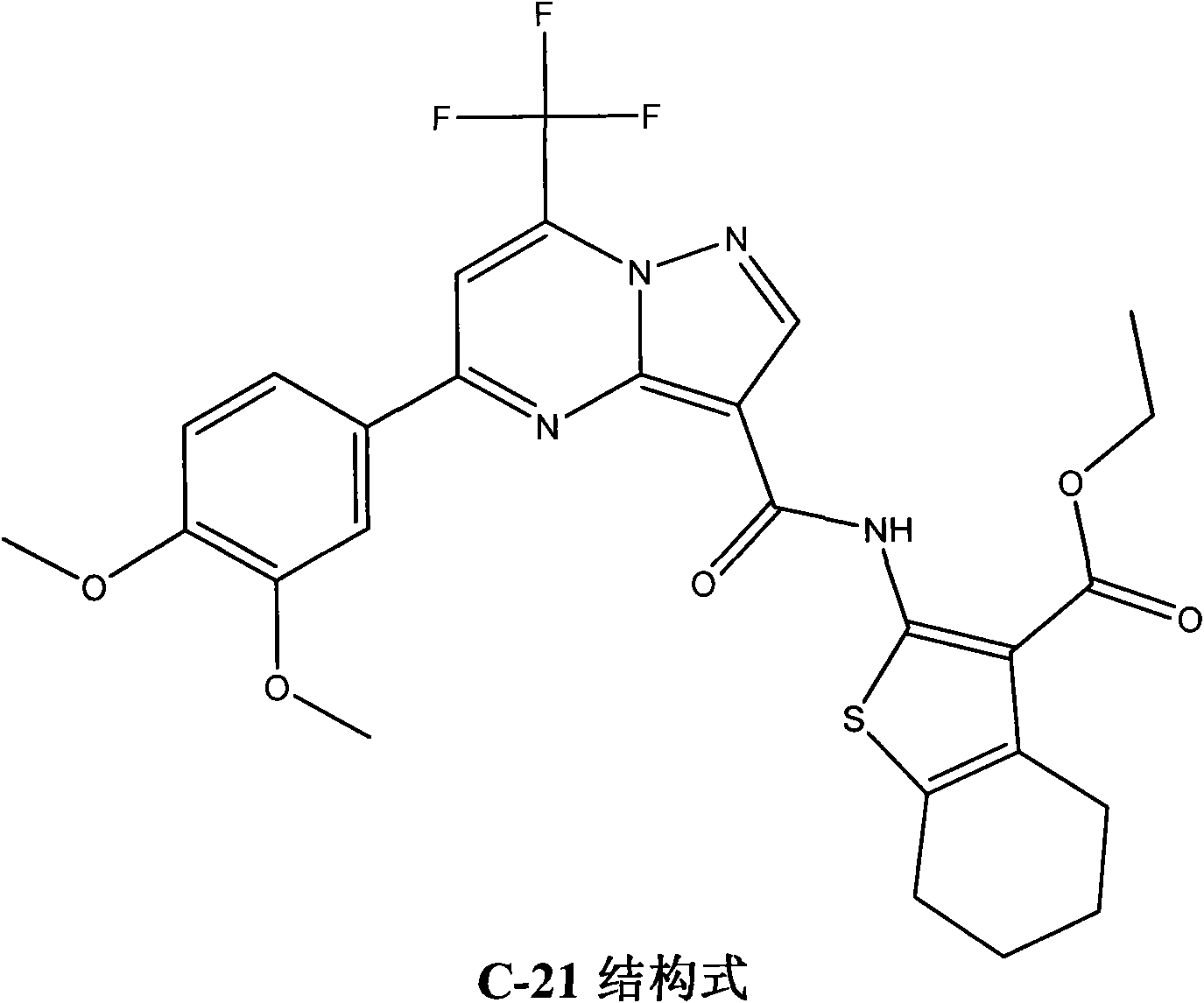
[0051] In this study, compound C-21 (a specific substituted derivative of N-(thiophene-2)pyrazolo[1,5-a]pyrimidine-3-carboxamide) with the highest inhibitory activity was selected for further molecular mechanism research .
[0052] H1299 cells in the logarithmic growth phase were taken, operated according to the instructions of RIPA cell lysate, and then subjected to ultrasound to obtain fresh cell lysate, which was quantified by BCA kit. Adjust the concentration of cell lysate and CTD protein solution to an appropriate level, add different concentrations of drug C-21, shake and mix (a certain amount of ATP solution can be added at the same time), react at 37°C for 4 hours, and take an appropriate amount for 10% SDS-PAGE electrophoresis. After the electrophoresis is completed, according to the gel area, press 0.65mA / cm 2 Turn on the power, and electrotransfer for 3 hours. After the transfer, the ...
Embodiment 3
[0054] Example 3: Binding mode of compound C-21 and CDK9 crystal structure
[0055] According to the known amino acid sequence of CDK9, on the SGI graphics workstation, the homology modeling (MODELLER / HOMOLOGY) module of the software Insight II was used to carry out the homology modeling of the three-dimensional structure of CDK9. The main steps are as follows: use the primary sequence of CDK9 as a probe, use the BlastP program to search for homologous proteins in the PDB library, and select homologous proteins with high homology and known protein types as templates. Superimposed with homologous proteins, the SCR (structurally conserved region) and LOOP regions were determined. Align the modeled protein and homologous protein sequences, the sequence that can match the SCR region of the homologous protein is the SCR region of the modeled protein, and then assign the spatial coordinates of the SCR region of the modeled protein, and then use the Modeler software package to query ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com