Recombinant human keratinocyte growth factor-2 containing eye drops and method for preparing same
A technology of keratinocytes and growth factors, applied in the field of recombinant human keratinocyte growth factor-2 eye drops and its preparation, can solve the problems of affecting eyesight, strong permeability, easy bleeding and secondary fibrosis, etc. The effect of stability and application convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] 1. Prescription Examples
[0011] prescription:
[0012] KGF-2 100μg
[0013] Glycerin 2g
[0014] Methionine 0.2g
[0015] Histidine hydrochloride 1g
[0016] EDTA0.2g
[0017] Tween 80 0.01g
[0019] Add 50mM phosphate buffer to 100ml
[0020] preparation:
[0021] Weigh the prescribed amount of glycerin, methionine, histidine hydrochloride, EDTA, Tween 80 and sodium chloride and dissolve in 60 ml of 50 mM phosphate buffer solution, and sterilize with flowing steam (115° C., 30 minutes). After cooling, add the prescribed amount of KGF-2 in a local 100-level environment, stir well, add 50mM phosphate buffer to about 90ml, adjust the pH to 7.0, and add 50mM phosphate buffer to 100ml. Filter through a 0.22μm filter membrane, and then pack.
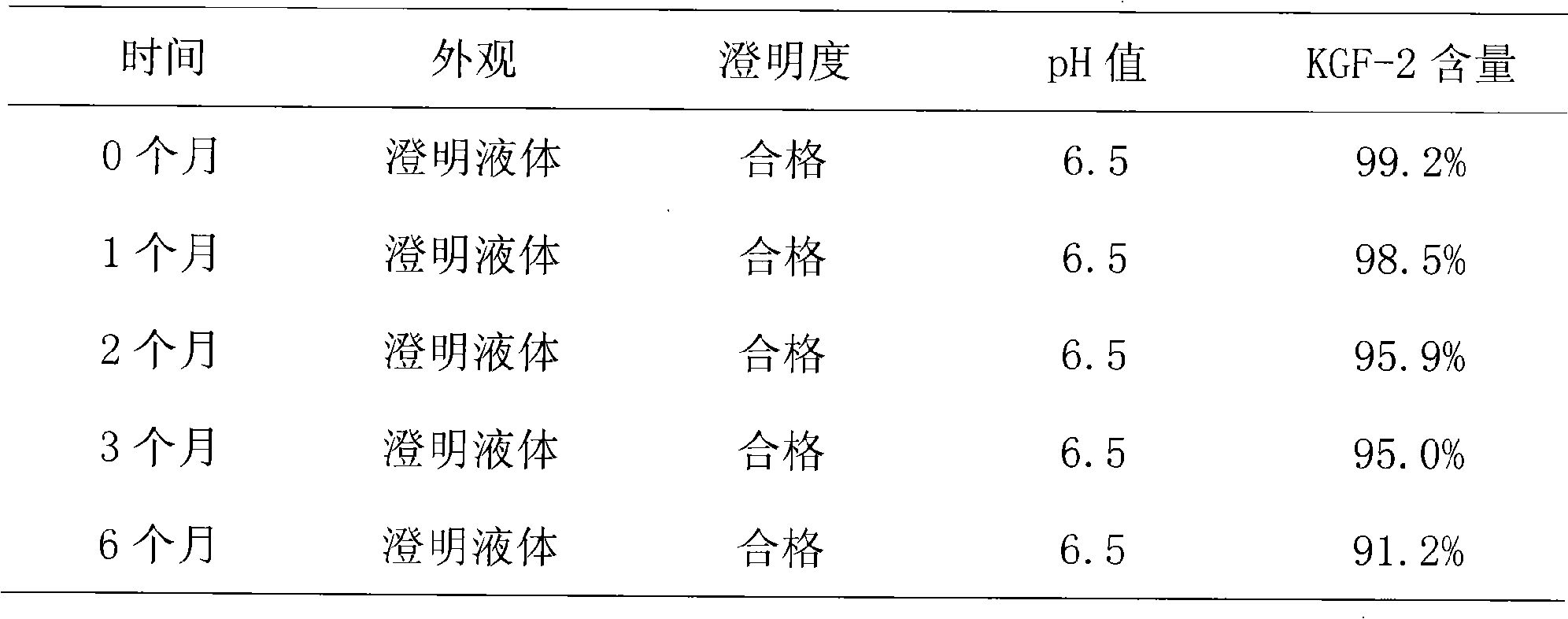
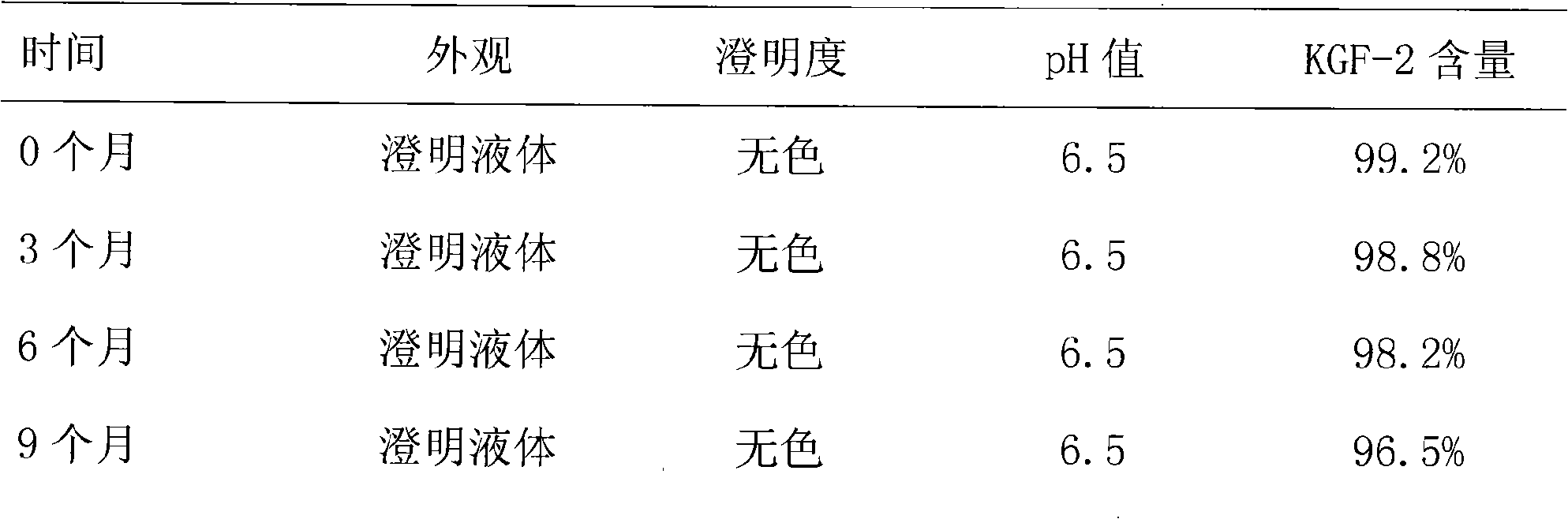
[0022] Stability experiment:
[0023] According to "Chinese Pharmacopoeia" (2005 edition), the accelerated stability test was carried out. Temperature 25℃±2℃, relative humidity 60%RH±5%. At ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com