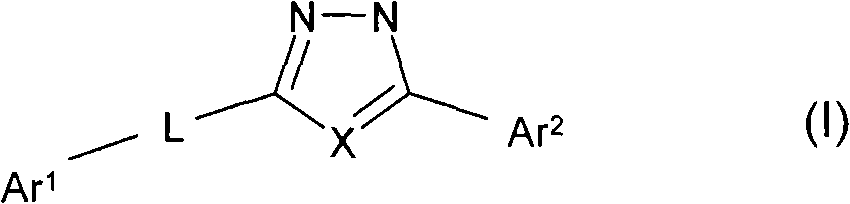
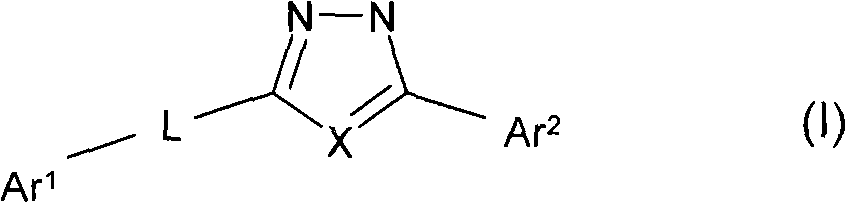
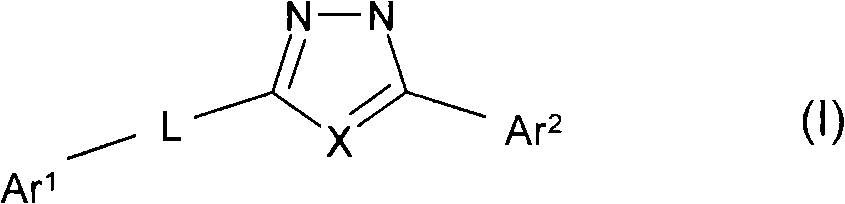
Oxadiazole and thiadiazole compounds and their use as nicotinic acetylcholine receptor modulators
A technology of compounds and mixtures, applied in the field of oxadiazolyl and thiadiazolyl derivatives, can solve problems such as not describing the activity of nicotinic receptor modulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] 5-furan-2-yl-[1,3,4]oxadiazole-2-thiol (intermediate compound)
[0241]
[0242] Dissolve 6.7g (0.12mole) of potassium hydroxide in 125ml of methanol, add 13.7g (0.11mole) of 2-furanoic hydrazide (2-furanoic hydrazide) and keep the temperature at 25°C for half an hour, then add 16.5g ( 0.22mole) of carbon disulfide. The reaction mixture was heated to reflux and stirred for 8 hours, then evaporated to an oil. Water was added to the residue, and concentrated hydrochloric acid was added until pH=4. The precipitate was isolated by filtration and dried. Yield 12.9 g (77 mmol, 70%).
[0243] In the same way, the following intermediate compound was similarly prepared: 5-pyridin-3-yl-[1,3,4]oxadiazole-2-thiol.
Embodiment 2
[0245] 2-(5-furan-2-yl-[1,3,4]oxadiazol-2-ylsulfanyl)-pyrazine (compound 2.1)
[0246]
[0247] 5-furan-2-yl-[1,3,4]oxadiazole-2-thiol (1.8 g, 11 mmole) was dissolved in 50 ml of dry dioxane, and 1.3 g (11 mmole) was added to the solution of chlorpyrazine and 1.4 g (11 mmole) of ethyldiisopropylamine. The reaction mixture was heated at reflux for 3 days and evaporated to an oil. 1 N (aq.) sodium hydroxide was added to the oil and the product was isolated by filtration and purified by column chromatography. Yield 1.1 g (4.5 mmole, 41%); Mp. 92-93°C.
[0248] With this, the following intermediate compounds are obtained similarly:
[0249] 3-(5-Benzylsulfanyl-[1,3,4]oxadiazol-2-yl)-pyridine (Compound 2.2) Mp.209-210°C; and
[0250] 3-Chloro-6-(5-furan-2-yl-[1.3.4]oxadiazol-2-ylsulfanyl)-pyridazine (Compound 2.3) Mp. 132-133°C.
Embodiment 3
[0252] N-(5-phenylmethanesulfonyl-[1,3,4]oxadiazol-2-yl)-acetamide (compound 3.1)
[0253]
[0254] To 2-acetylamino-5-benzylthio-[1,3,4]thiadiazole (3 g, 11 mmole) in dichloromethane was added 8.6 g (50 mmole) of 3-chloroperoxybenzoic acid. The reaction mixture was stirred at room temperature for 90 minutes and filtered. The filtrate was evaporated to an oil. The oil was titrated with water and a white precipitate formed which was isolated by filtration, washed with water and dried. Yield 2.6 g (8.7 mmole, 79%); Mp. 245-248°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com