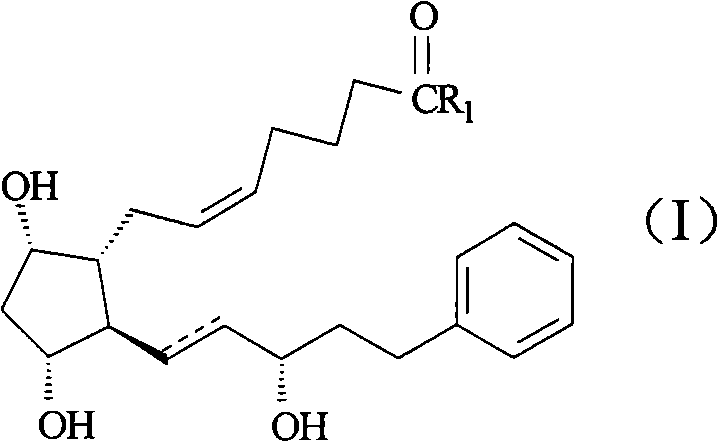
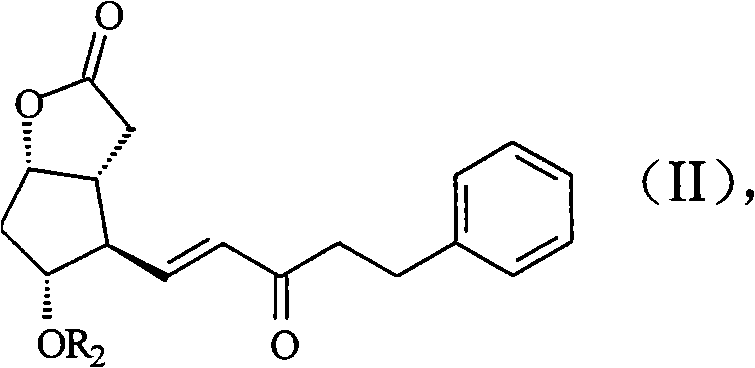
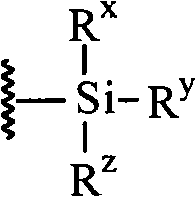Producing method of prostaglandin F-type derivant
A manufacturing method and compound technology, which are applied in the manufacturing field of prostaglandin F-type derivatives and can solve problems such as loss of vision, constricted visual field, damage to optic nerve and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153]
[0154] Add 6.71g of lithium chloride (LiCl) and 30ml of tetrahydrofuran (THF) into a 250ml three-necked reaction flask, dropwise add the THF solution of compound (XII) (9.01g of (XII) compound is dissolved in 40ml of THF), and in -15 At ~-5°C, a THF solution of triethylamine (TEA) was added dropwise (dissolve 9.88ml of triethylamine in 20ml of THF). After stirring for 30 min, the THF solution of compound (XI) was added dropwise (10 g of compound (XI) was dissolved in 40 ml THF), reacted for 1 hour, and detected by thin layer analysis sheet (TLC sheet). After the reaction, 100 ml of water was added at room temperature to stop the reaction, and 100 ml of ethyl acetate was used to extract twice, and the upper layer was taken and sodium sulfate was added to remove water. The filtrate obtained by filtering off sodium sulfate was concentrated under reduced pressure to obtain an oily substance, and was separated by column chromatography with solvent ethyl acetate / hexane=1...
Embodiment 2
[0158]
[0159] Add 12.05g of compound (II-1) and 120ml of THF into a 500ml three-neck reaction flask, and add 54.26g of (-)-chlorodiisopinepyrylborane ((-) dropwise at -60°C to -75°C -chlorodiisopinocamphenylborane) solution (ie 62.5% in heptane), naturally warmed up and stirred for 15hrs. After the end of the reaction, add 80ml of saturated aqueous sodium bicarbonate (NaHCO 3(aq) ), and adjust the pH=6 to 7, then add 80ml of ethyl acetate to extract 3 times, take the upper layer and add 100ml of water to wash. After taking the upper layer liquid and adding sodium sulfate to remove water, the filtrate obtained by filtering off sodium sulfate was concentrated under reduced pressure to obtain 47.3 g of an oily crude product. The crude product was then extracted by adding 180 ml of hydrogen methane (CAN) and 85 ml of hexane (Hexane). After standing to separate layers, the lower layer was removed and extracted three times by adding 85 ml of hexane. After extraction, the lowe...
Embodiment 3
[0168]
[0169] Get 1.13g of compound (II-1) and 11ml of acetone into a 50ml reaction flask, add dropwise 19% aqueous hydrochloric acid (HCl (aq) ) and adjust the pH3(aq) The pH was adjusted to 6-7, and after concentration under reduced pressure, 10 ml of ethyl acetate was added to extract 3 times. After standing for stratification, sodium sulfate was added to the upper layer to remove water, and after sodium sulfate was filtered off, the filtrate was concentrated under reduced pressure to obtain 1.04 g of oily crude product (XIII). The crude product (XIII) is then directly subjected to the next step reaction.
[0170] 1.04 g of crude product (XIII), 10 ml of catalytic amount of p-toluenesulfonic acid (PTSA) and dichloromethane (dichloromethane; CH 2 Cl 2 ) into a 100ml reaction flask, and 0.47ml dihydropyran (DHP) was added dropwise, stirred for 1 hour, and whether the reaction was complete was detected by TLC chips. After the reaction is complete, add 10ml of water to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com