Stable liposomal formulations for ocular drug delivery
A kind of liposome preparation, the technology of liposome, be used in the field of liposome preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of large unilamellar vesicles (LUV) for drug release studies
[0028] A thin-film hydration technique was used to formulate latanoprost-loaded POPC liposomes for drug release rates. Briefly, POPC was weighed and dissolved in a chloroform:methanol (2:1 v / v) solvent mixture. Latanoprost (2 mg / ml stock solution in acetonitrile) was added to the lipid solvent mixture at a drug:lipid molar ratio of 0.175:1 and maintained at 40°C. The solvent mixture was added to a round bottom flask in a rotary evaporator attached to a water bath maintained at 40°C. The flask was rotated at 100 rpm for 1 hour under low pressure to remove solvent, thereby forming a thin drug-loaded lipid film. Isotonic phosphate buffered saline (PBS) (150 mM, pH 5.5) was added to this membrane to form multilamellar vesicles (MLV). These MLVs were extruded 10 times through polycarbonate filters (0.2 μm-0.08 μm). This extrusion resulted in the formation of LUVs with diameters having...
Embodiment 2
[0029] Example 2: Preparation of LUVs for Drug Stability Studies
[0030] Drug-loaded LUVs were also prepared for stability studies. POPC was dissolved in PBC (at pH 6.7) and kept stirring at room temperature for 2 hours to form MLV. These MLVs were extruded 3-5 times using 3 polycarbonate films of 80 nM size stacked together on a benchtop extruder to form POPC LUVs. Latanoprost was dissolved in ethanol, and the resulting solution (in a round bottom flask maintained in a water bath at 50°C) was dried under a stream of nitrogen to form a thin drug film. The drug film was hydrated with the POPC LUVs at room temperature for 2-3 hours until no oil droplets were observed on the flask walls. A latanoprost:POPC molar ratio of 0.175:1 was used to prepare drug-loaded LUVs. Latanoprost-loaded POPC LUVs were sterile filtered at room temperature using a 0.2 μm syringe filter and stored at 4°C until analysis. A similar method was used to prepare eggPC liposomes loaded with latanopros...
Embodiment 3
[0031] Example 3: Characterization of drug-loaded POPC liposomes
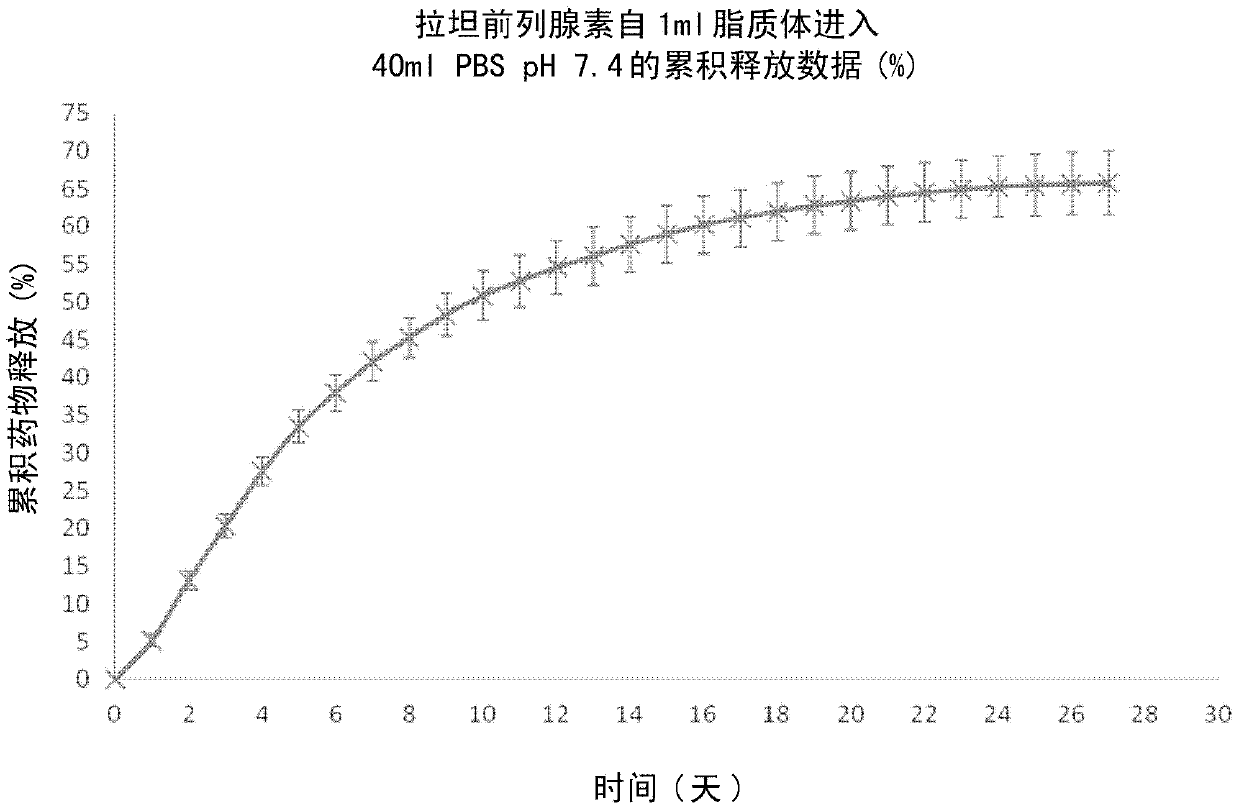
[0032] Drug release studies were performed by dialyzing latanoprost-loaded POPC liposomes prepared as described in Example 1 above against PBS (at pH 7.4) and measuring The amount of latanoprost released. The results are shown in figure 1 middle. Over a period of 28 days, approximately 65% of latanoprost was released from the latanoprost-loaded POPC liposomes in a time-dependent manner.
[0033] The physical characteristics of these latanoprost-loaded POPC liposomes were measured essentially as described in International Application Publication No. 2012 / 021107 by Venkatraman et al., the contents of which are incorporated herein in their entirety as as reference data.
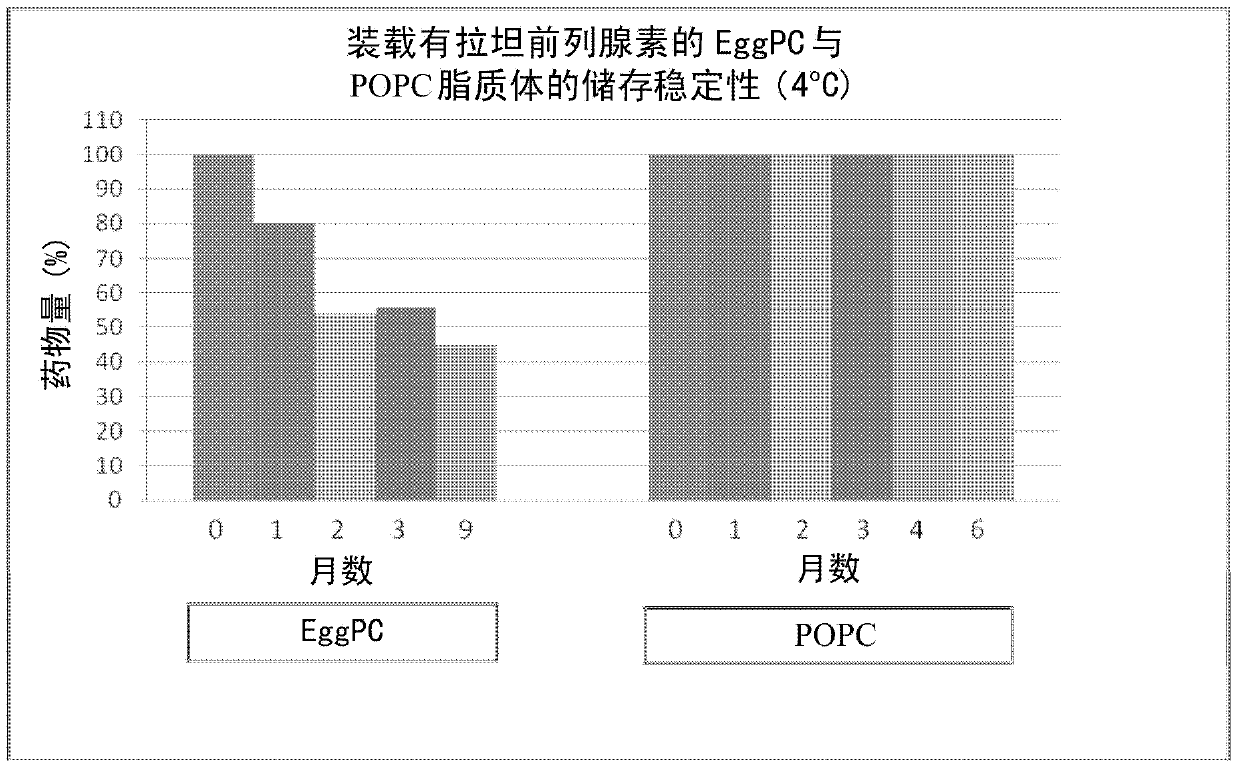
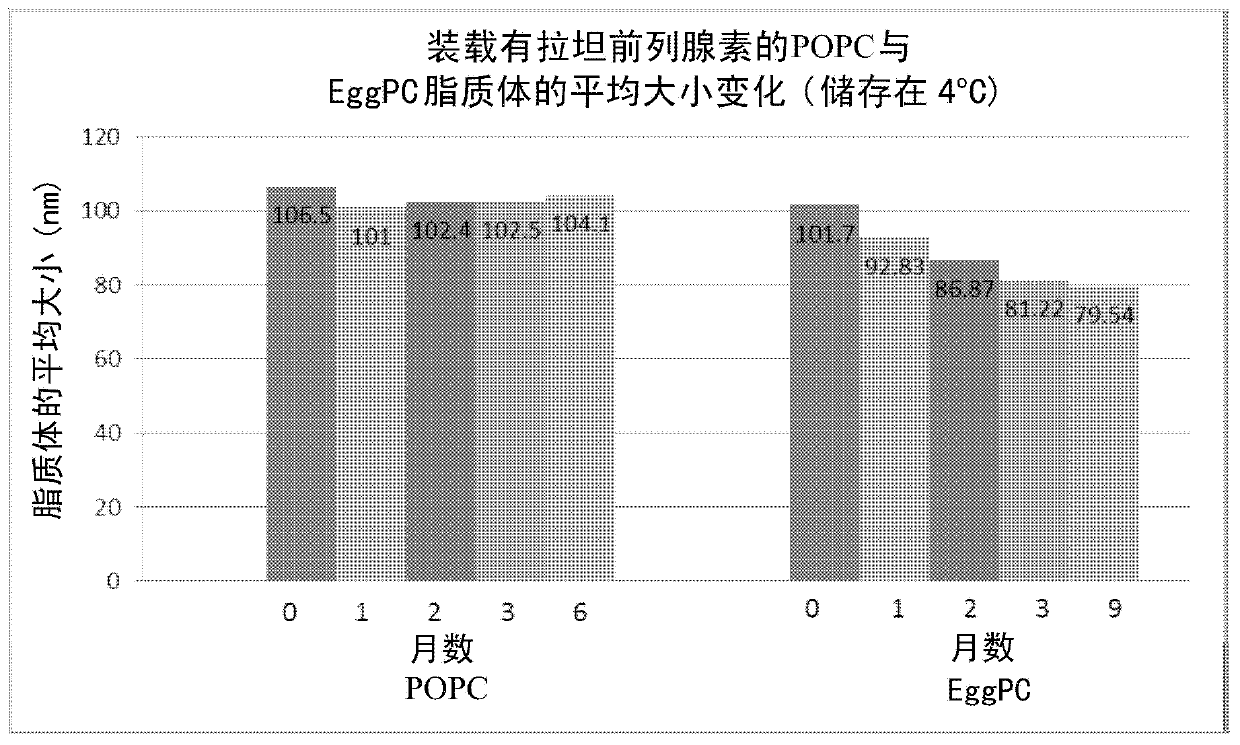
[0034] The stability of latanoprost-loaded POPC liposomes prepared as described in Example 2 above was measured and compared to similar latanoprost-loaded eggPC liposomes. The stability of these liposomes stored at 4°C was assessed over a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com