Novel encochleation methods, cochleates and methods of use
a technology of encochlease and cochlease, which is applied in the direction of instruments, salicyclic acid active ingredients, saccharide peptide ingredients, etc., can solve the problems of added manufacturing and product costs, affecting the activity and/or stability of encochleated materials, and time-consuming, so as to achieve efficient and easy scaling up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Amphotericin B Cochleate (CAMB) Prepared with DMSO and Lipid:AmB Ratios of 10:1, 2:1 and 1:1 w / w
Amphotericin B cochleates (CAMB) were prepared with Soy PS and DMSO with Vitamin E, and a Lipid to AmB ratio of 10:1 as follows.
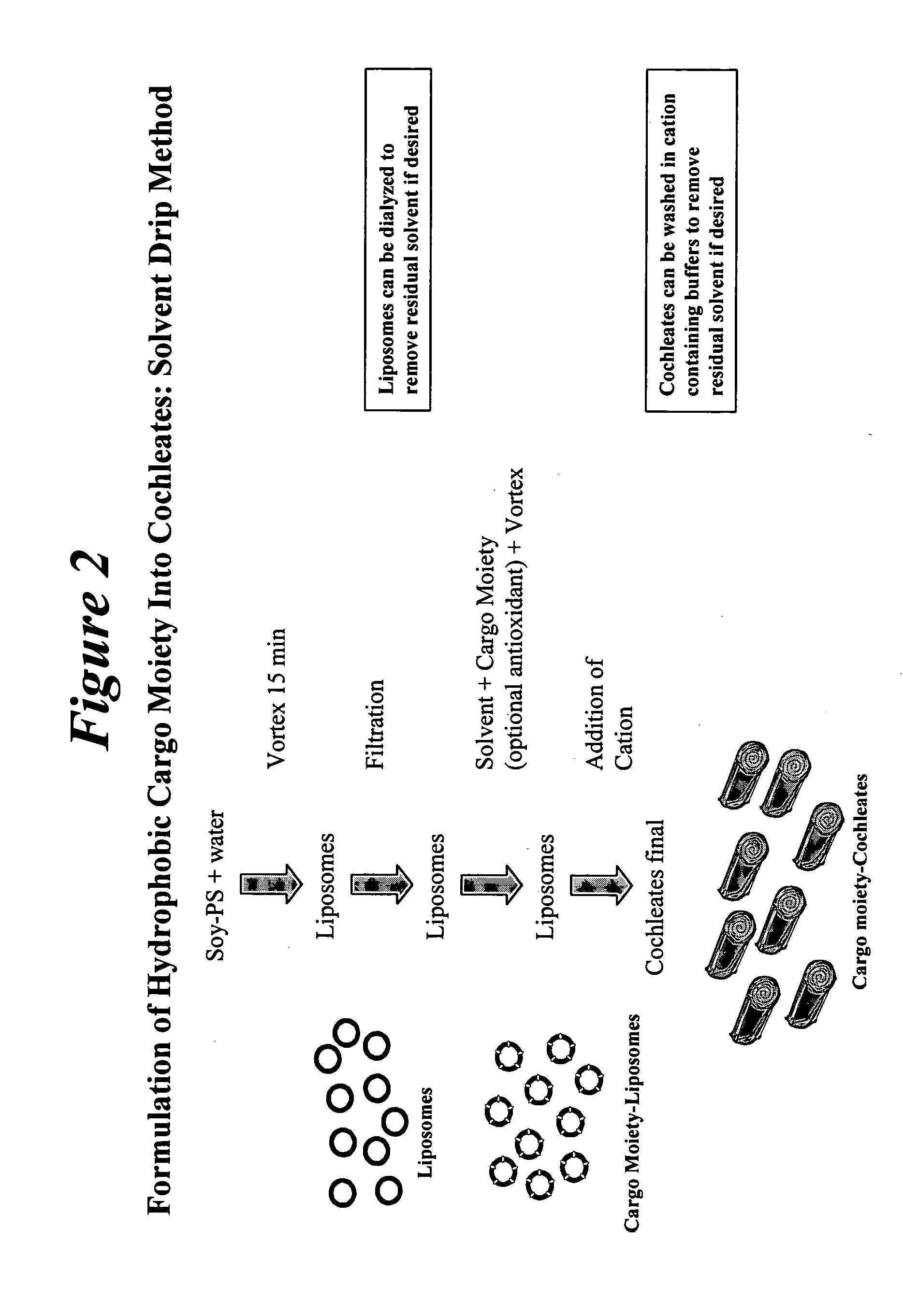
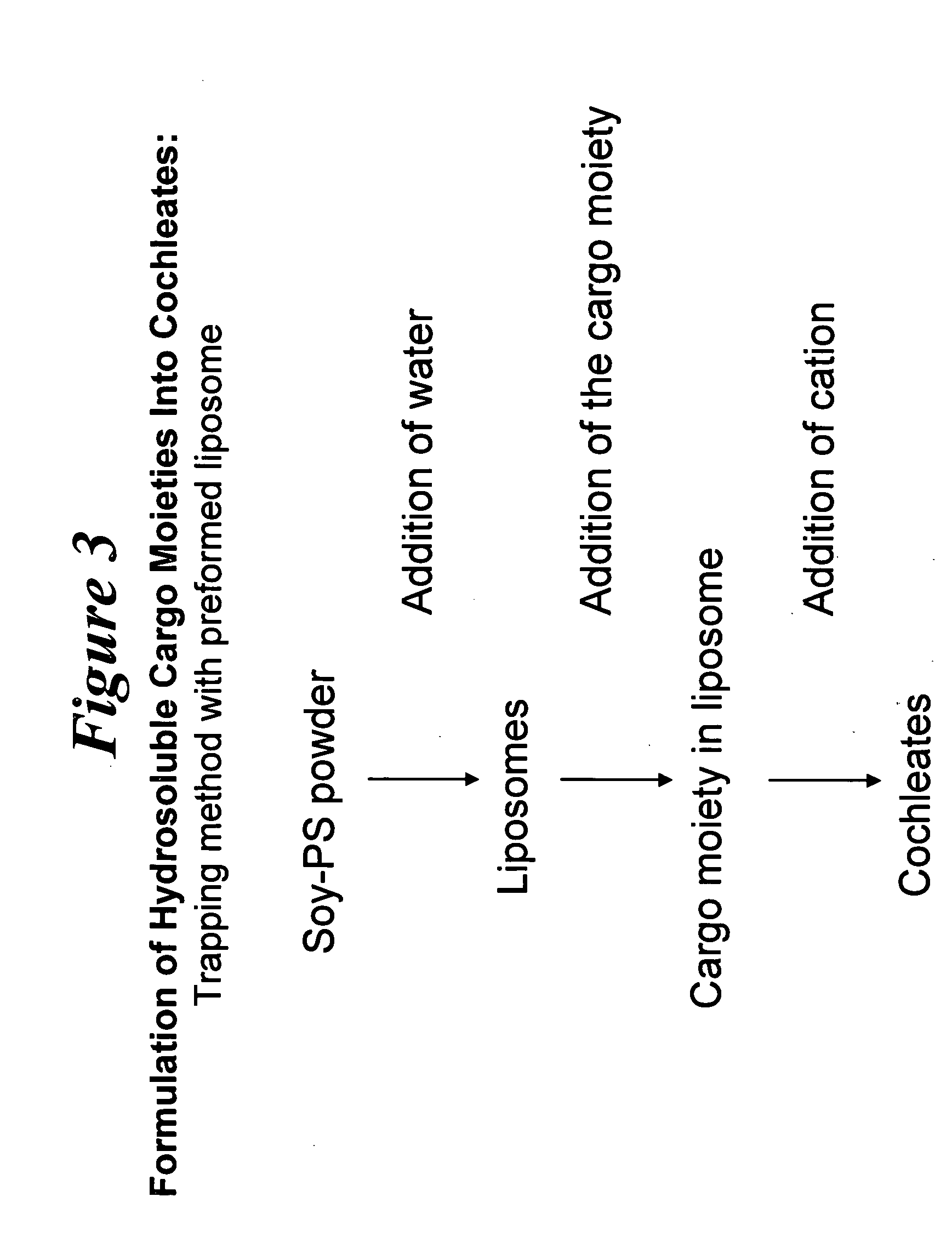
Preparation of Liposomes
20 ml of water was added to 200 mg of Soy-PS in a 50 ml plastic tube, vortexed for about 15 minutes to form a liposomal suspension, and filtered using a 0.45 μm filter. The suspension was sonicated for about 4 minutes and filtered again with a 0.22 μm filter.
Addition of Cargo Moiety and Antioxidant in Solvent
1.90 ml of DMSO solvent was added to 20 mg of Amphotericin B in a 15 ml plastic tube. To the AmB / DMSO mixture, 0.1 ml of Vitamin E (20 mg / ml in DMSO), vortexed for about 10 minutes. This solution was then added to the liposomal suspension by drop wise addition using a 1 ml pipette while vortexing. The final mixture was vortexed for about 2 minutes.
Precipitation of Cochleates
2 ml of calcium (0.1 N) was added to the liposom...
example 2
Amphotericin B Cochleates Prepared with NMP
Cochleates were prepared as described in Example 1, except N-methylpyrrolidone (NMP) solvent was used instead of DMSO, and the formulation was adjusted as indicated in the following table.
TABLE 2NMP 10:1 FormulationCAMB / NMP 10:1Soy PS (mg)200AmB (mg)20Vitamin E (mg)2DMSO (Ml)2Water (ml)20Calcium (ml)2Washings with sterile water w / 2calcium (1 mM)Final Volume20ml
Cochleates in the final formulations were observed as a yellowish suspension. Mice infected with a lethal dose of Aspergillus fumigatus were dosed with 2 mg / kg of the AmB-cochleate formulation for 14 days. The cochleates were efficacious against the A. fumigatus.
example 3
Amphotericin B Cochleates Prepared with DMSO and Lipid:AmB Ratios of 5:1, 4:1, 3:1 and 2:1 w / w
Amphotericin B cochleates (CAMB) were prepared with soy PS and DMSO with tocopherol (Vitamin E) with the following protocol: 1. Weighing and placing 300 mg of soy PS into a 50 ml pp sterile tube with 10 ml sterile water. 2. Vortexing the suspension for 2 minutes. 3. Sonicating the suspension for 3 minutes. 4. Filtering the suspension with a 0.22 μm filter and pooling liposomes into a 50 ml tube. 5. Weighing and placing 10 mg (5:1), 12.5 mg (4:1) 16.6 mg (3:1) and 25 mg (2:1), of AmB (individually) into four 15 ml pp sterile tubes with 0.5 ml DMSO and vortexing. 6. Adding 6.0 μl (5:1), 6.2 μl (4:1), 6.6 μl (3:1), and 7.5 μl (2:1) of tocopherol at 10 mg / ml in DMSO to the 15 ml tubes with AmB. (The concentration of the AmB was 20 mg / ml (5:1), 25 mg / ml (4:1), 33.2 mg / ml (3:1), and 50 mg / ml (2:1), at this time) 7. Vortexing the solution for a few minutes until the AmB completely dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size distribution | aaaaa | aaaaa |
| Size distribution | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com