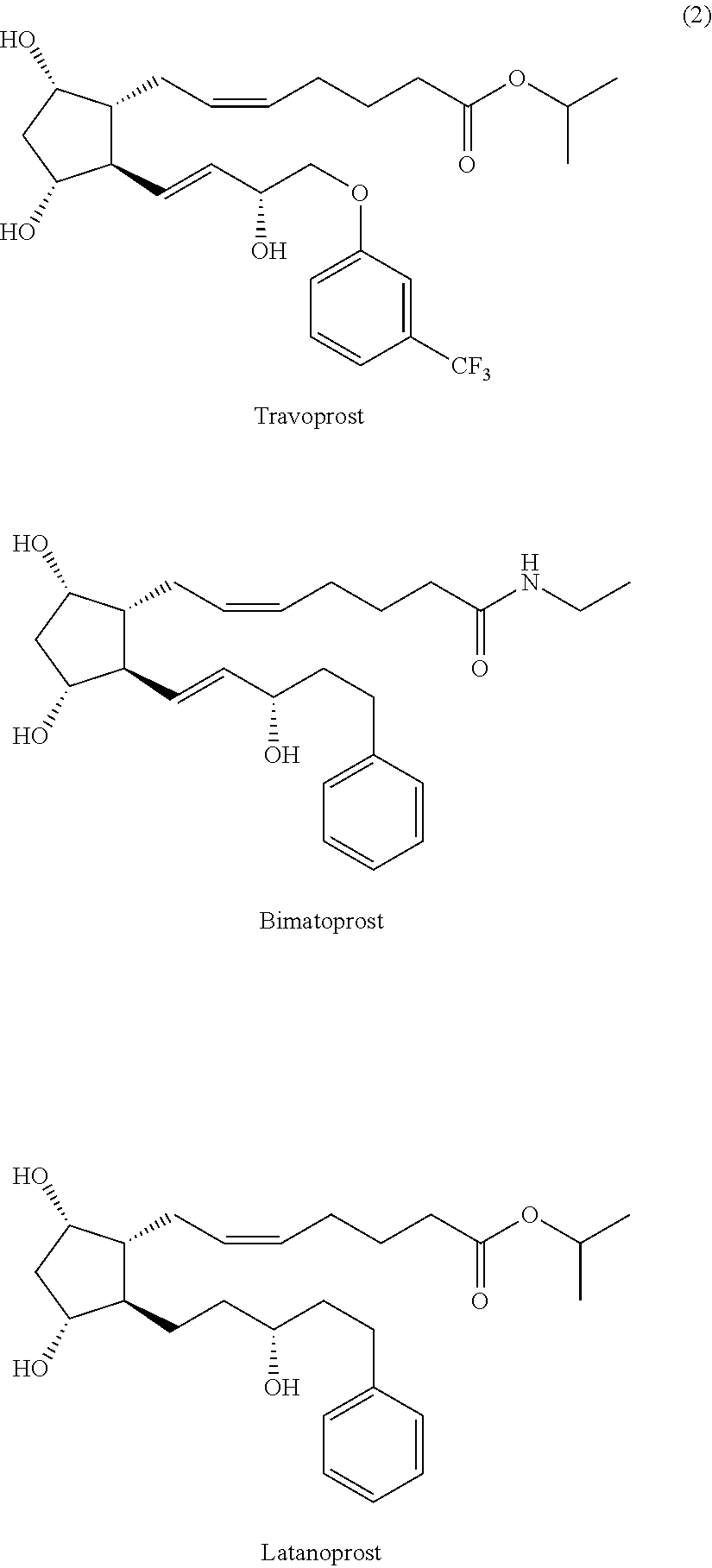
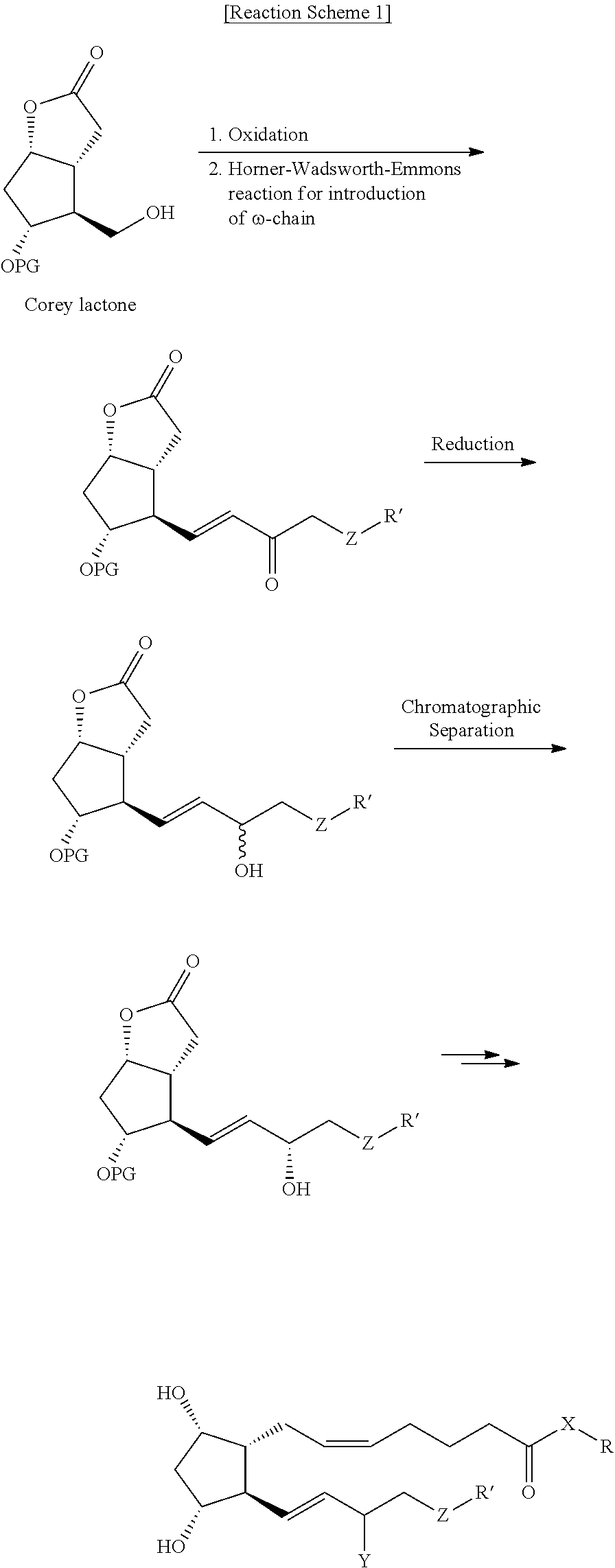
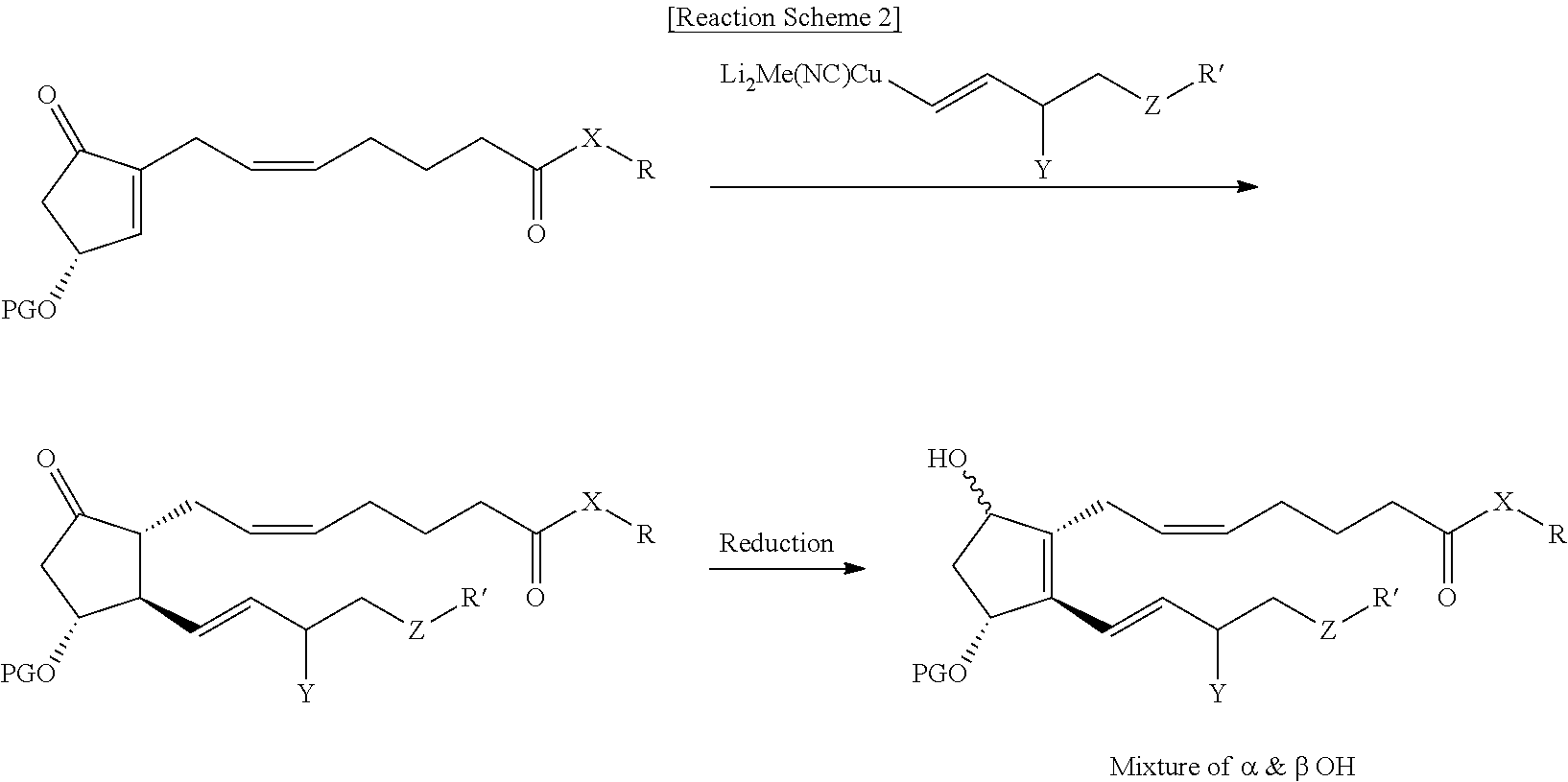
Process for preparing prostaglandin derivatives
a prostaglandin and derivative technology, applied in the field of process for efficiently preparing prostaglandin derivatives, can solve the problems of inconvenient process, high cost of corey lactone, and inability to large-scale production of prostaglandin derivatives, and achieve the effect of high purity and efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Compound (6-I)
[0047]
[0048]Pyridinium p-toluensulfonate (PPTS, 2.3 g) was added to compound (8) (127 g) dissolved in a mixture of acetone (1.2 L) and water (0.25 L), followed by stirring at room temperature for 12 hours. After the reaction was completed, the resulting reaction solution was concentrated under vacuum, and ethyl acetate (1.5 L) and water (1 L) were added thereto, followed by stirring. The organic layer was separated, dried over sodium sulfate (1 kg), filtered and concentrated. The resulting residue was subjected to chromatography (eluent: n-hexane:ethyl acetate=1:3) to give the target compound (78 g, Yield: 89%).
example 3
Preparation of Travoprost
[0049]
[0050]2,6-Di-tert-butyl-4-methyl phenol (172 g) was dissolved in toluene (2 L), followed by cooling to 0° C., and DIBAL (1.0 M toluene, 625 ml) was added dropwise thereto for 1 hour. The resulting reaction solution was cooled to −70° C., and compound (6-I) (78 g) dissolved in toluene (0.5 L) was added dropwise thereto. The resulting reaction solution was stirred for about 2 hours, and its temperature was slowly raised to −40 to −20° C., followed by stirring for 4 hours. After the reaction was completed, an aqueous 2N hydrochloric acid solution (1 L) was added. The organic layer was separated, dried over sodium sulfate (1 kg), filtered and concentrated. The resulting residue was subjected to chromatography (eluent: n-hexane:ethyl acetate=1:5) to give travoprost (Purity: 96% or more). The obtained compound was subjected to preparative HPLC (eluent: dichloromethane:isopropanol=90:10) to give highly pure travoprost (50 g, Purity: 99.5% or more, Yield: 63%)...
example 4
Preparation of Compound (9)
[0051]
[0052]Copper cyanide (98 g) was dissolved in THF (2.2 L), followed by cooling to 0° C., and methyllithium (1.6 M diethyl ether, 1.44 L) was added dropwise thereto. The resulting reaction solution was stirred for 10 to 20 minutes, and compound (3-II) (598 g) dissolved in THF (1.4 L) was added thereto. The resulting reaction solution was stirred for 1.5 to 2 hours, followed by cooling to −70° C., and compound (4-II) (270 g) dissolved in THF (2.2 L) was added thereto for 15 minutes, and then the temperature of the reaction solution was slowly raised to −45° C. After the reaction was completed, the resulting reaction solution was added to a mixture of aqueous ammonium chloride solution / ammonia water (9:1, 7.0 L) and diethyl ether (3.5 L), followed by stirring at room temperature for 1 to 2 hours. The organic layer was separated, dried over sodium sulfate (1 kg), filtered and concentrated. The resulting residue was subjected to chromatography (eluent: n-h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com