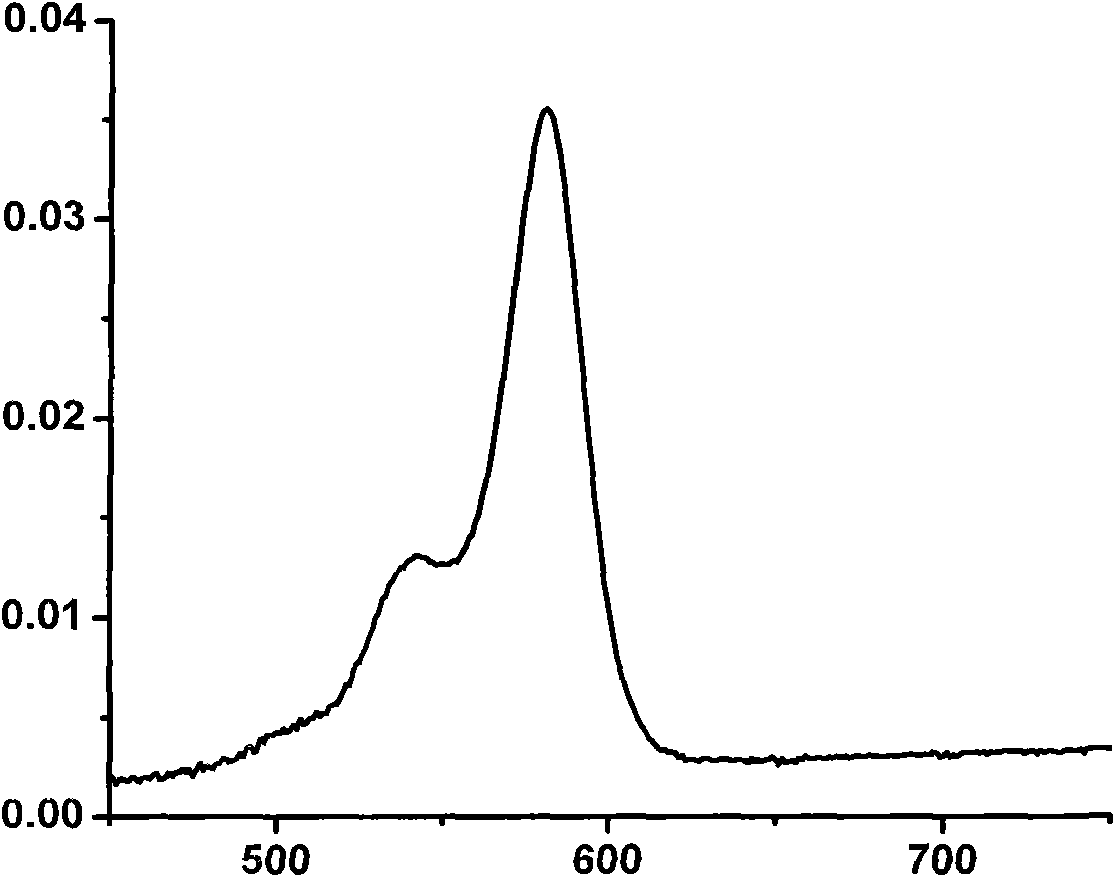
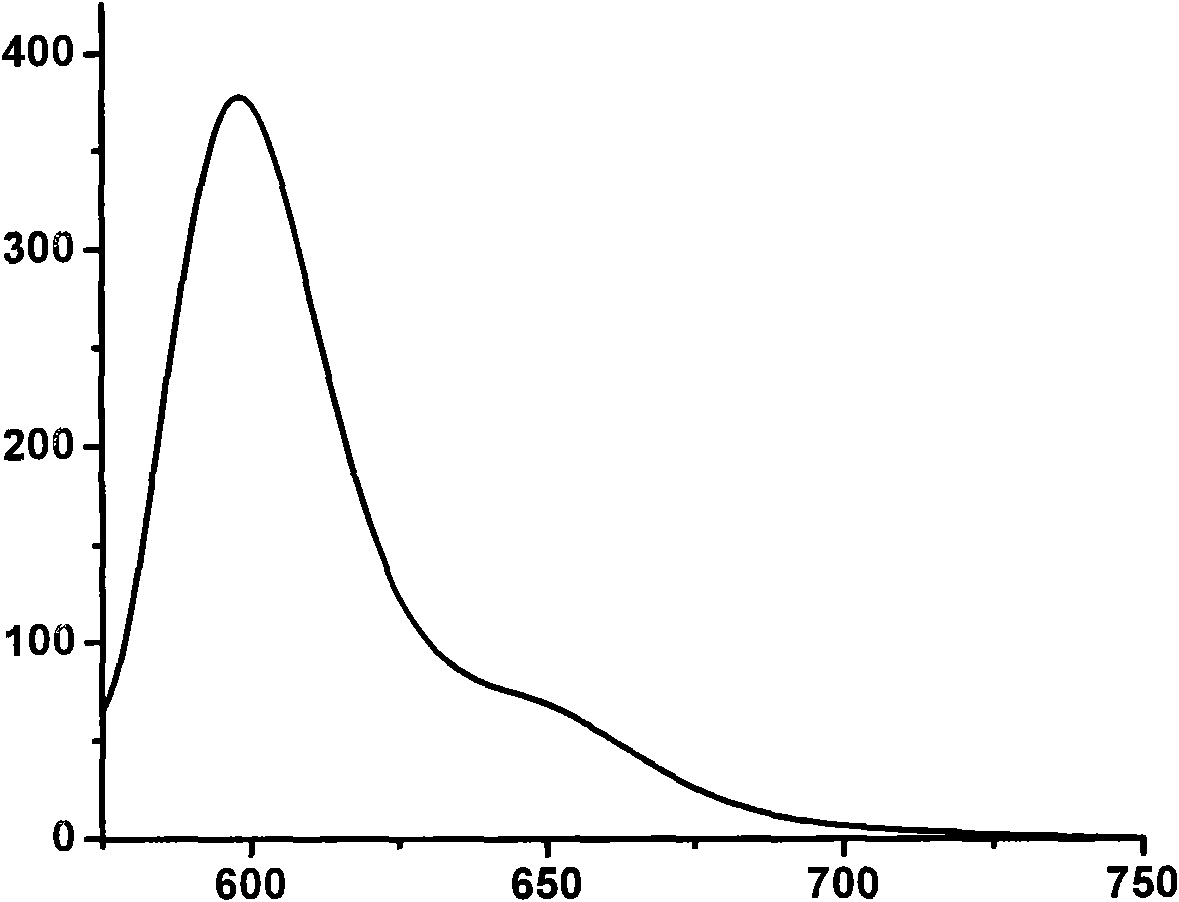
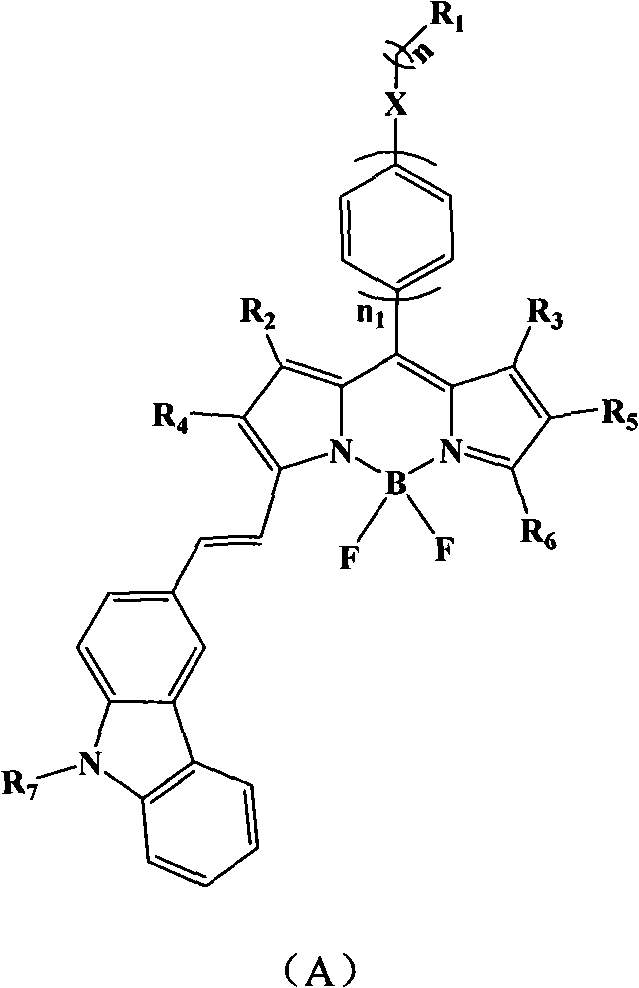
Strong-fluorescence boron dipyrromethene dye containing carbazole structure
A technology of fluorescent fluoroboron dipyrrole and fluoroboron dipyrrole, which is applied in the directions of organic dyes, azo dyes, luminescent materials, etc., can solve the problems of difficulty, difficulty in laser dyes, and reduction of laser efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039]In a 100mL two-necked bottle, add 0.50mmol of fluoroborate dipyrrole raw material, 3 times the molar amount of N-butylcarbazole aldehyde, and 0.1mmol of piperidine as catalyst, 3 capsules Molecular sieves were ground into powder and added to the system, 20mL o-dichlorobenzene, under the protection of nitrogen, magnetically stirred, kept the system at 120°C, reacted for 8h, passed through a silica gel column, and obtained a blue-purple solid with a yield of 70%, HRMS [M] + : 557.2817(m / z); 1 H-NMR (400Mz, CDCl3): δ=1.36(s, 3H), 1.70(s, 3H), 2.56(s, 3H), 3.4(t, 2H), 5.5(s, 1H), 5.6(s, 1H), 6.84 (d, 2H) 7.21-7.30 (m, 12H).
Embodiment 2
[0041]
[0042] In a 100mL two-necked bottle, add 0.50mmol of fluoroborate dipyrrole raw material, 6 times the molar amount of N-ethylcarbazole aldehyde, and 0.1mmol of piperidine as catalyst, 3 capsules Molecular sieves were ground into powder and added to the system, 20mL of N-methylpyrrolidone, under nitrogen protection, magnetically stirred, kept the system at 120°C, reacted for 16h, passed through a silica gel column, and obtained a dark green solid with a yield of 35%, HRMS [M] + : 780.3926(m / z); 1 H-NMR (400Mz, CDCl 3 ): δ = 1.36 (s, 6H), 1.70-2.54 (m, 15H), 3.4 (m, 4H), 6.84 (d, 4H), 7.21-7.30 (m, 14H).
Embodiment 3
[0044]
[0045] Add 0.50mmol fluoroborate dipyrrole raw material, 3.5 times the molar amount of N-isopropylcarbazole aldehyde, and 0.08mmol piperidine as catalyst in 100mL two-necked bottle, 3 capsules Molecular sieves were ground into powder and added to the system, 15mL of chlorobenzene, under the protection of nitrogen, magnetically stirred, kept the system at 130°C, reacted for 12h, passed through a silica gel column, and obtained a blue-purple solid with a yield of 65%, HRMS [M] + : 671.3491(m / z) 1 H-NMR (400Mz, CDCl 3 ): δ=1.36(s, 3H), 1.70(s, 3H), 1.85(t, 6H), 2.56(s, 3H), 3.4(m, 1H), 6.84(d, 2H) 7.21-7.30(m , 11H), 9.56 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com