Method for preparing dichloropropanol by glycerin chlorination
A technology of dichloropropanol and glycerin, applied in the direction of introducing halogen preparation, chemical recovery, organic chemistry, etc., can solve the problems of catalyst loss, long reaction time, low activity, etc., and achieve improved utilization rate, fast reaction speed, and improved yield rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
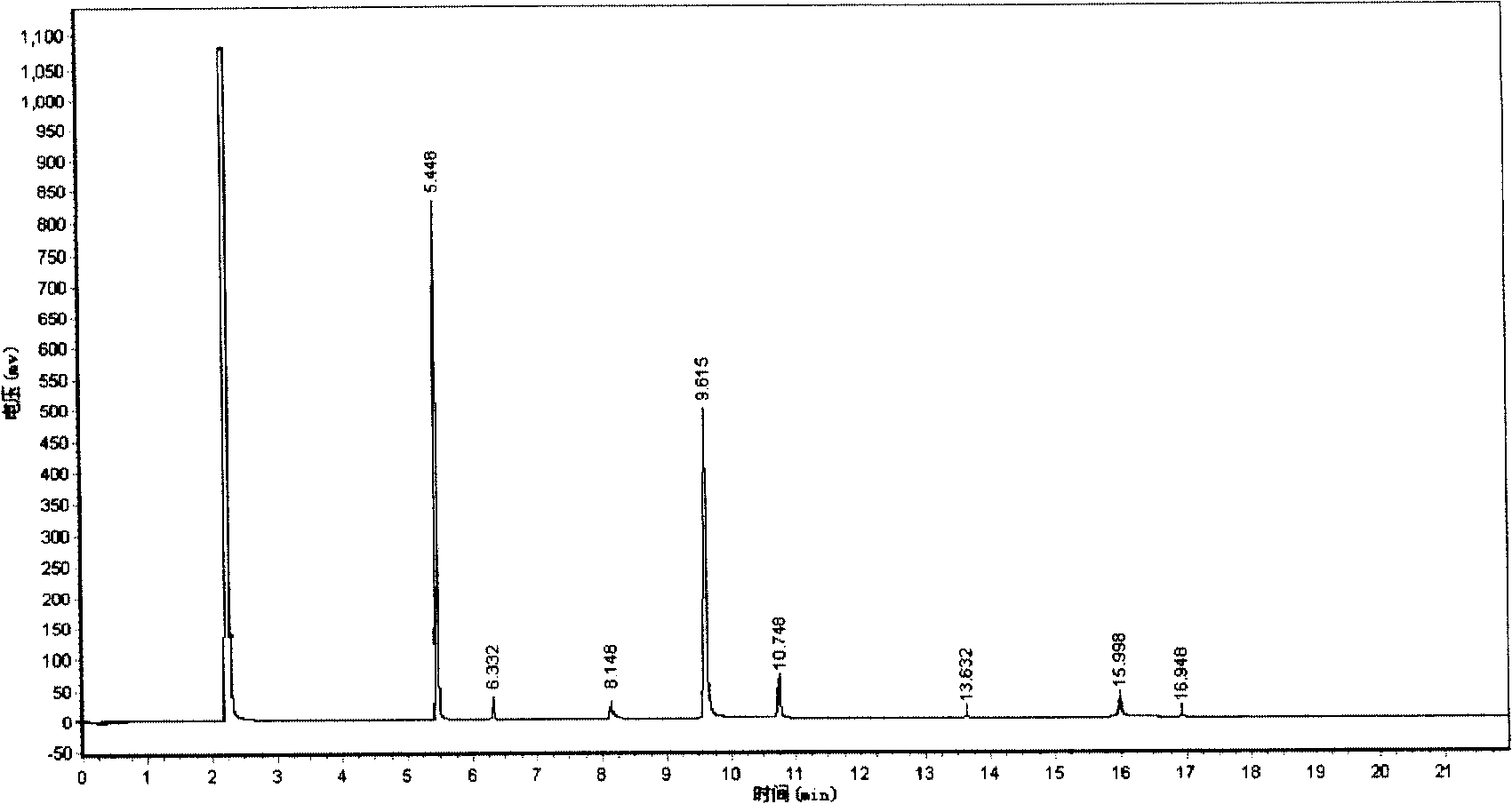
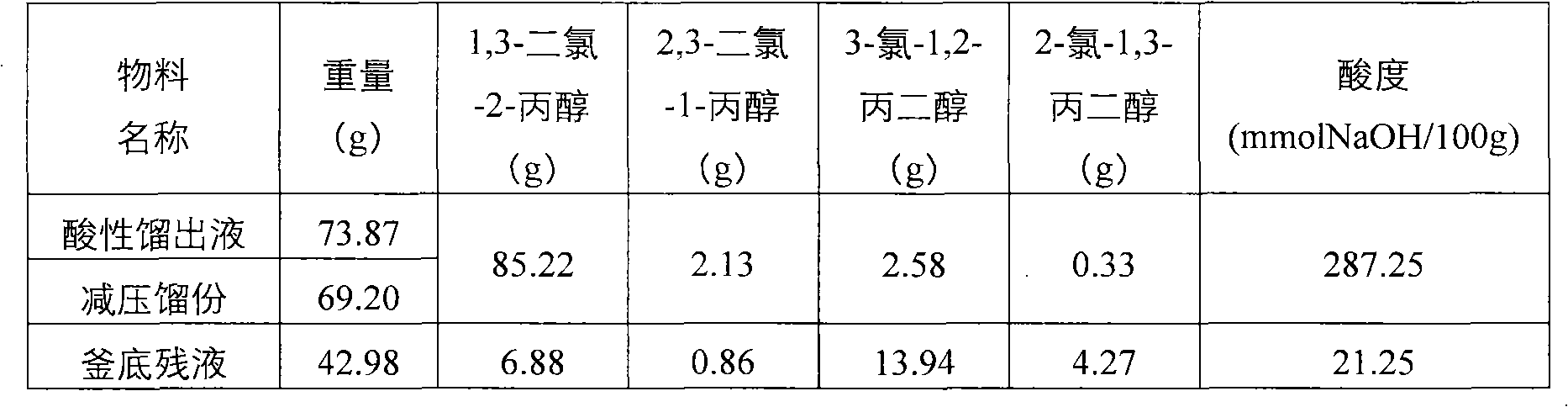
[0025] In a three-neck flask with a stirring and reflux device, add 92.05g (1.00mol) of glycerin and 11.43g (0.10mol) of cyclopentyl carboxylic acid (also known as cyclopentyl formic acid, English name: cyclopentanecarboxylic acid, provided by Bailingwei Chemical Reagent Company) , heated to 110°C, and hydrogen chloride gas was introduced, and the flow rate of hydrogen chloride gas was 200ml / min. After reacting for 8 hours, 112.18 g of chlorinated liquid was obtained, and 73.87 g of acidic distillate was obtained by reflux during the reaction, and the conversion rate of glycerol was 99.68% through chromatographic analysis. The chlorinated liquid was distilled under reduced pressure to obtain 69.20 g of reduced-pressure fractions and 42.98 g of bottom liquid. The acidic distillate and the reduced-pressure fractions were combined. Obtain the total yield of dichloropropanol by chromatographic analysis: 73.76%, the total yield of monochloropropanediol: 19.13%, and the reaction pro...
Embodiment 2
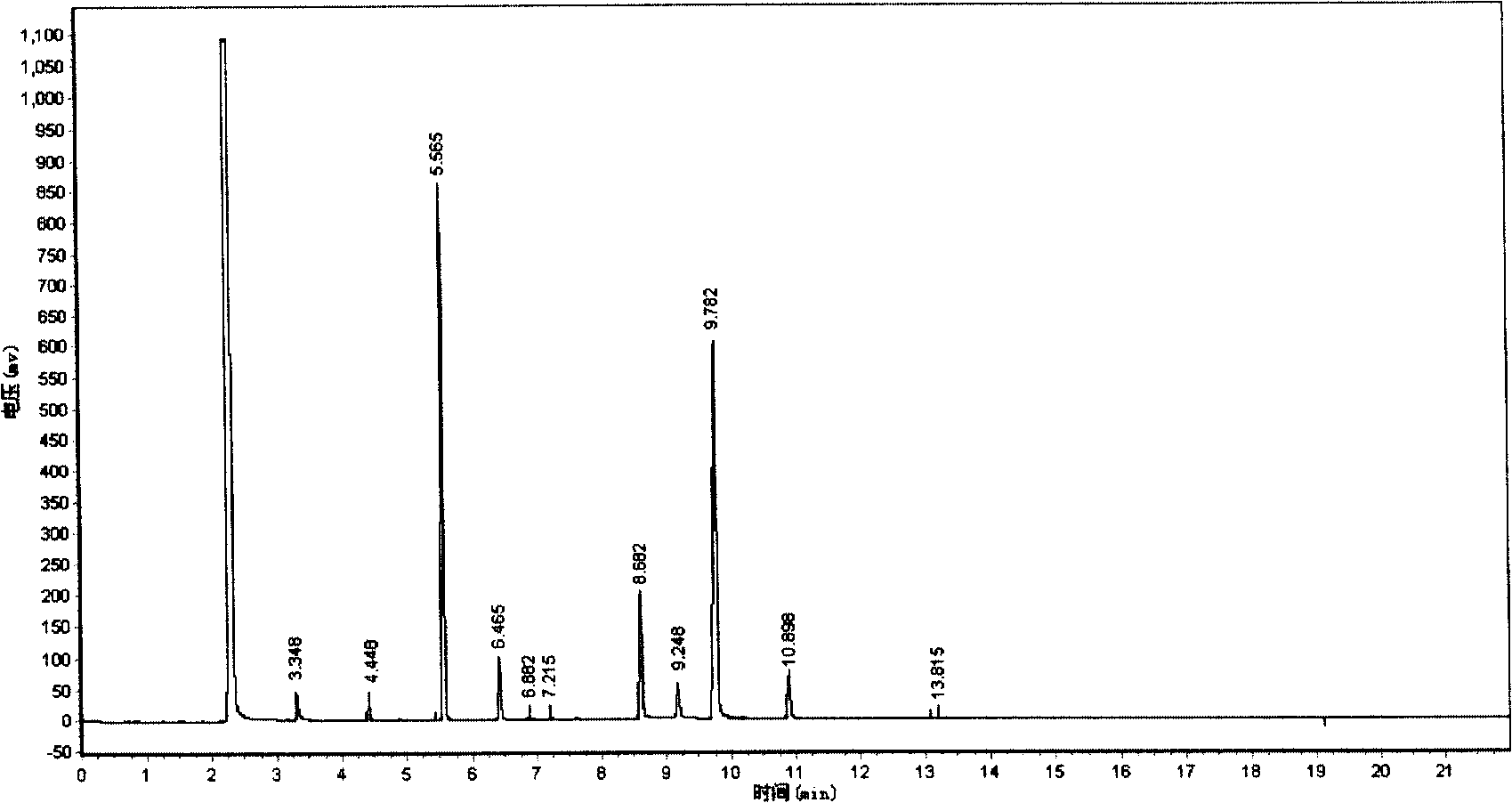
[0029] In a there-necked flask with a stirring and reflux device, add 92.25g (1.002mol) glycerin and the 42.98g still bottom residue obtained in Example 1, heat to 110°C, feed hydrogen chloride gas, and the flow rate of hydrogen chloride gas is 200ml / min. After reacting for 8 hours, 146.32 g of chlorinated liquid was obtained. During the reaction, reflux was condensed to obtain 68.90 g of acidic distillate, and the conversion rate of glycerin was 99.58% through chromatographic analysis. The chlorinated liquid was distilled under reduced pressure to obtain 94.35 g of reduced-pressure fractions and 51.97 g of bottom liquid. The acidic distillate and the reduced-pressure fractions were combined. Obtain the total yield of dichloropropanol by chromatographic analysis: 82.61%, the total yield of monochloropropanediol: 15.28%, and the reaction product composition sees the following table 2:
[0030] Table 2
[0031]
[0032]
Embodiment 3
[0034] In a there-necked flask with a stirring and reflux device, add 92.21g (1.002mol) of glycerin and the 51.97g residue at the bottom of the still obtained in Example 2, heat to 110°C, feed hydrogen chloride gas, and the flow rate of hydrogen chloride gas is 200ml / min. After reacting for 8 hours, 161.02 g of chlorinated liquid was obtained. During the reaction, reflux was condensed to obtain 68.60 g of acidic distillate, and the conversion rate of glycerin was 99.49% through chromatographic analysis. The chlorinated liquid was distilled under reduced pressure to obtain 101.85 g of reduced-pressure fractions and 59.17 g of bottom liquid. The acidic distillate and the reduced-pressure fractions were combined. Obtain the total yield of dichloropropanol by chromatographic analysis: 87.91%, the total yield of monochloropropanediol: 11.88%, and the composition of the reaction product is shown in the following table 3:
[0035] table 3
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com