Method for preparing flavonoid dimeric compound as well as medium and preparation method thereof
A technology for flavonoids and intermediates, which is applied in the new synthesis field, can solve the problems of long route, complicated and tedious operation, low total yield and the like, and achieves the effects of simple operation, high yield and short route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
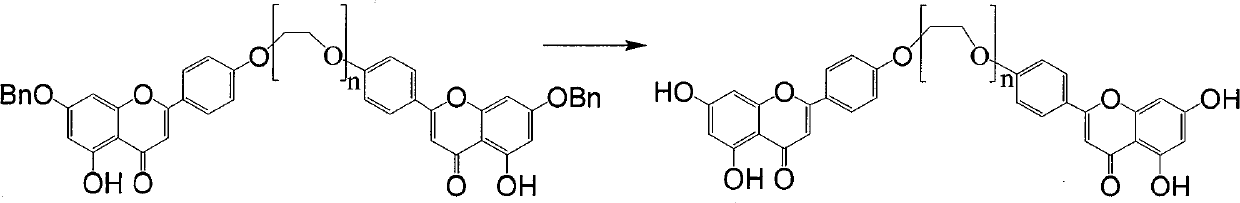
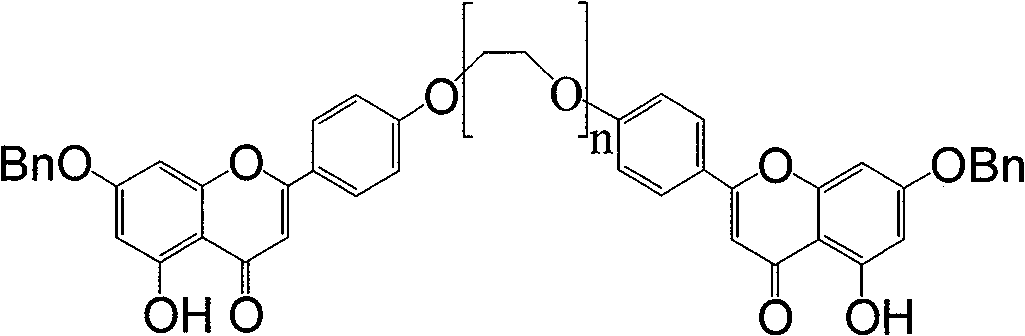
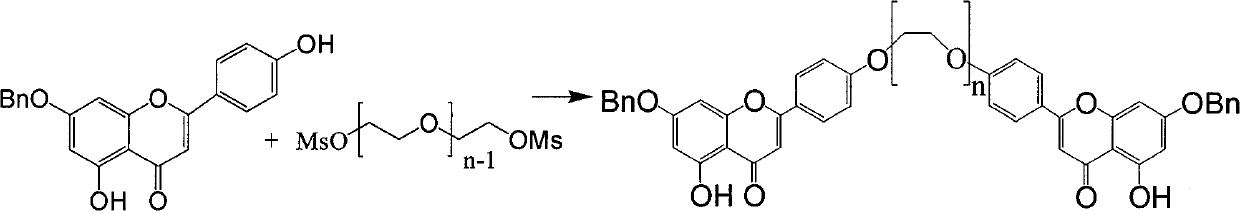
[0055] Example 1 1,13-two (7-benzyl-5,4 '-dihydroxypigenin)-1,4,7,10,13-pentaoxo-tridecane (formula A compound (n= 4))
[0056] 7-benzyl-5,4'-dihydroxypigenin (142mg, 0.42mmol) was dissolved in dimethylformamide 3ml, potassium carbonate (283mg, 2.06mmol) was added, after stirring for ten minutes, formula C (n= 4, 72mg, 0.21mmol), react at 140~150°C for 3 hours (TLC detects that Formula C is consumed), cool, add 10ml of water, wash the water phase with dichloromethane (10ml×3), collect the dichloromethane phase and use saturated Wash the dichloromethane phase (20ml×3) with brine, dry over anhydrous sodium sulfate, collect the filtrate by filtration, and concentrate the filtrate to obtain a yellow solid formula A (n=4, 106mg) by column chromatography (dichloromethane:methanol=50:1) , 58%).
[0057] 1 H NMR (400MHz, CDCl3) δ: 3.68(m, 8H); 3.87(t, 4H); 4.16(t, 4H); 5.11(s, 4H); 6.40(d, 2H); 6.50(d, 4H) ;6.52(s,2H);6.97(d,4H);7.25(m,10H);7.75(d,4H);12.74(s,2H)
[0058] 13 C N...
Embodiment 2
[0060] Example 2 1,13-two (7-benzyl-5,4 '-dihydroxypigenin)-1,4,7,10,13-pentaoxo-tridecane (formula A compound (n= 4))
[0061] 7-benzyl-5,4'-dihydroxypigenin (142mg, 0.42mmol) was dissolved in dimethylacetamide 6ml, potassium carbonate (566mg, 4.06mmol) was added, and after stirring for ten minutes, formula C (n= 4, 110mg, 0.31mmol), react at 145-155°C for 3 hours (TLC detects that Formula C is consumed), cool, spin off acetone, add 10ml of water, wash the water phase with dichloromethane (10ml×3), collect dichloromethane Wash the dichloromethane phase (20ml×3) with saturated brine after the phase, dry over anhydrous sodium sulfate, filter and collect the filtrate, and column chromatography (dichloromethane:methanol=50:1) after the filtrate is concentrated to obtain a yellow solid formula A(n =4, 92 mg, 50%).
[0062] MS: 901 (M+Na)
Embodiment 3
[0063] Example 3 1,13-two (7-benzyl-5,4 '-dihydroxypigenin)-1,4,7,10,13-pentaoxo-tridecane (formula A compound (n= 4))
[0064] 7-benzyl-5,4'-dihydroxypigenin (142mg, 0.42mmol) was dissolved in 3ml of nitromethane, potassium carbonate (283mg, 0.42mmol) was added, after stirring for ten minutes, formula C (n=4, 72mg, 0.21mmol), react at 140-150°C for 3 hours (TLC detects that formula C is consumed), cool, add 10ml of water, wash the water phase with dichloromethane (10ml×3), collect the dichloromethane phase and wash with saturated saline The dichloromethane phase (20ml×3) was dried over anhydrous sodium sulfate, and the filtrate was collected by filtration. After the filtrate was concentrated, column chromatography (dichloromethane:methanol=50:1) gave yellow solid formula A (n=4, 87mg, 48 %).
[0065] MS: 901 (M+Na)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com