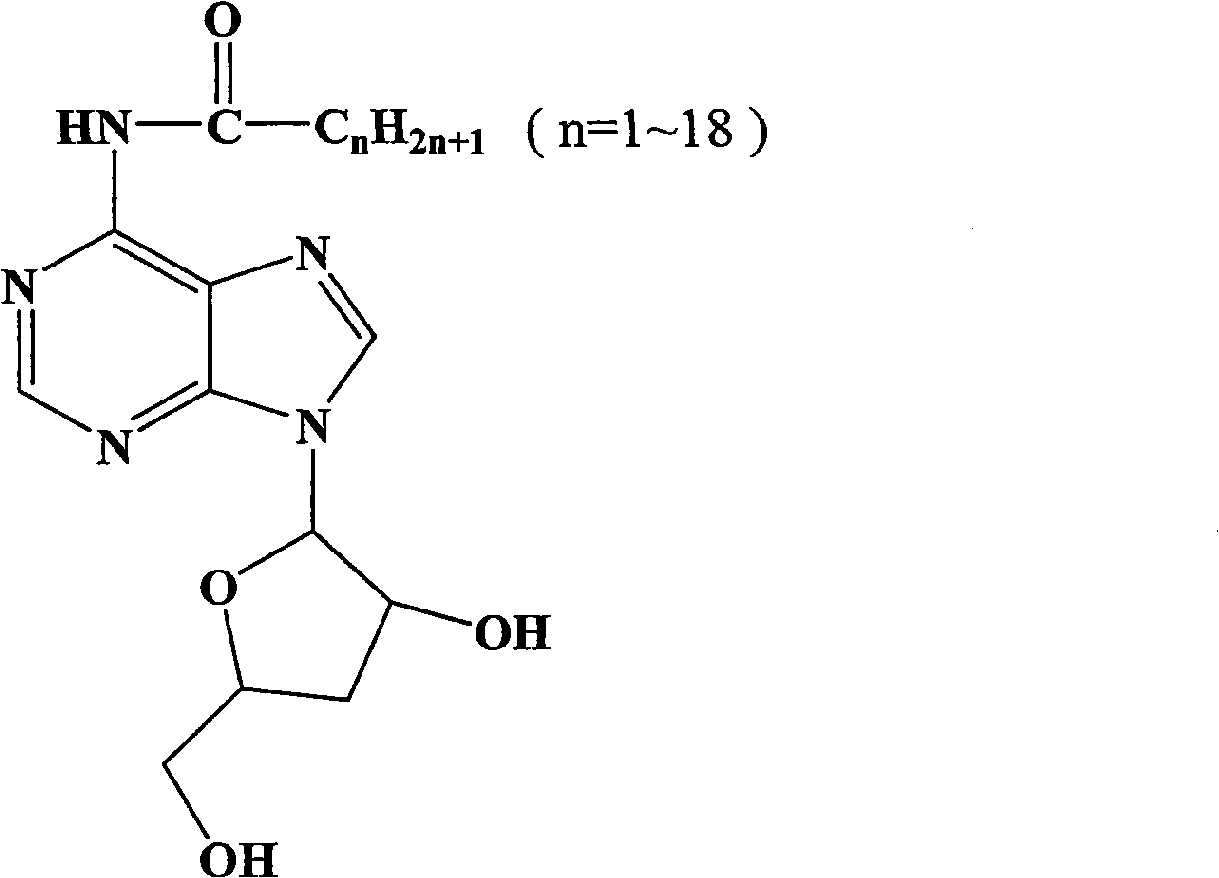
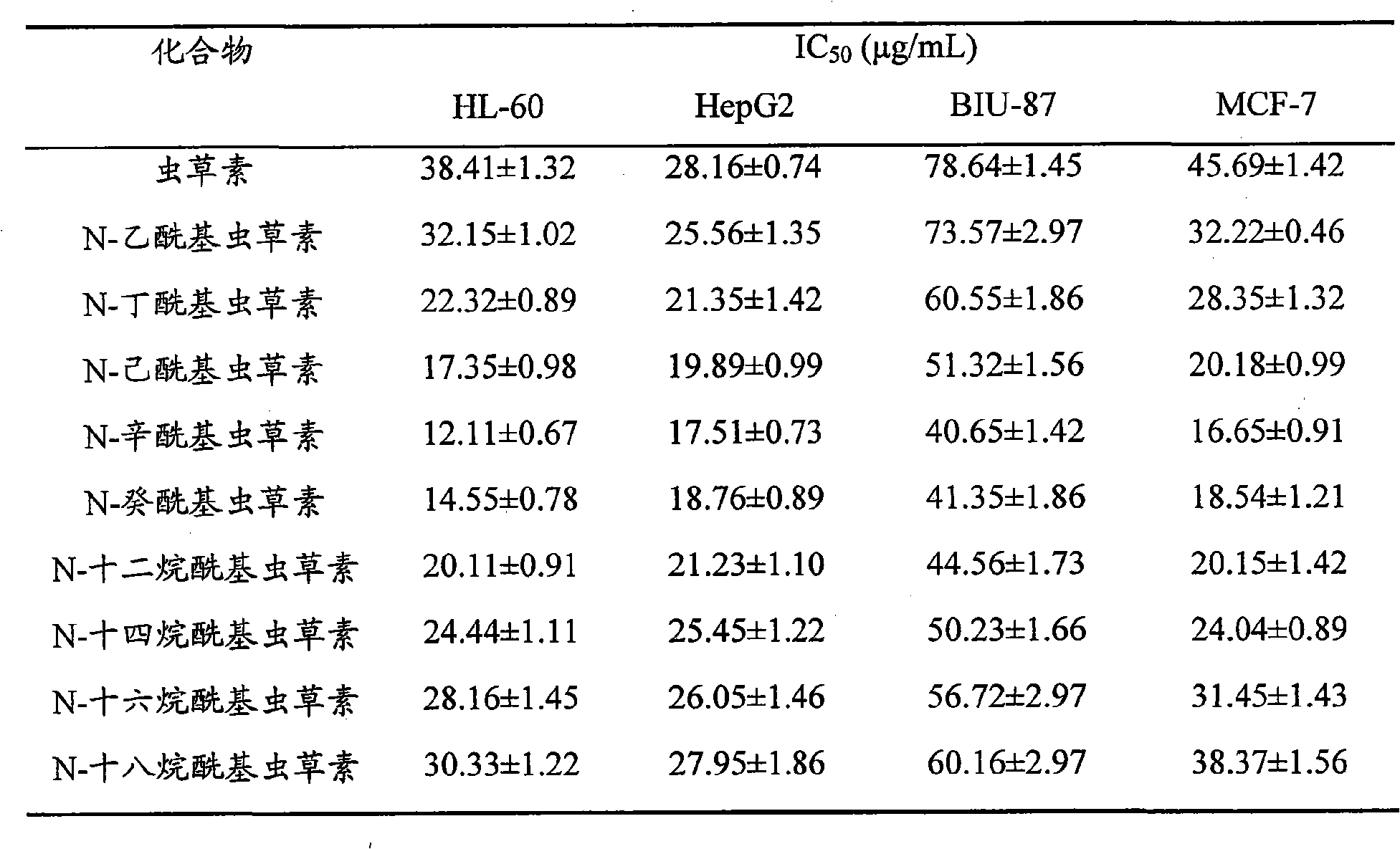
Application of N-alkanoyl cordycepin in preparing drugs for treating tumor diseases
A technology of alkanoyl cordycepin and tumors, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc. It can solve the problems of low practical value and achieve good application prospects and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] In order to make the purpose, technical solution and advantages of the present invention clearer, the antitumor activity of N-alkanoyl cordycepin is described in detail below through experimental examples.
[0013] Experimental method: (1) Reagent preparation: trypsin solution with a concentration of 0.25% (w / v): dissolve 1 g of trypsin in 100 mL of water, filter and sterilize, and dilute with 4×D-Hanks solution to the desired concentration before use. required concentration, and with a concentration of 5.6% (w / w) NaHCO 3 Adjust the pH to 7.2; tetramile salt (MTT) solution with a concentration of 5 mg / mL: dissolve 250 mg of MTT in 50 mL of PBS with a concentration of 0.01 mol / L and a pH of 7.4, filter and sterilize, and store at 4°C in the dark;
[0014] (2) Preparation of cell culture medium: RPMI-1640 medium dry powder 10.4g, NaHCO 3 Dissolve 2.0g, sodium pyruvate 110.0mg, L-glutamine 293.0mg, glucose 3.8g, penicillin 200,000 units, streptomycin 200,000 units and ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com