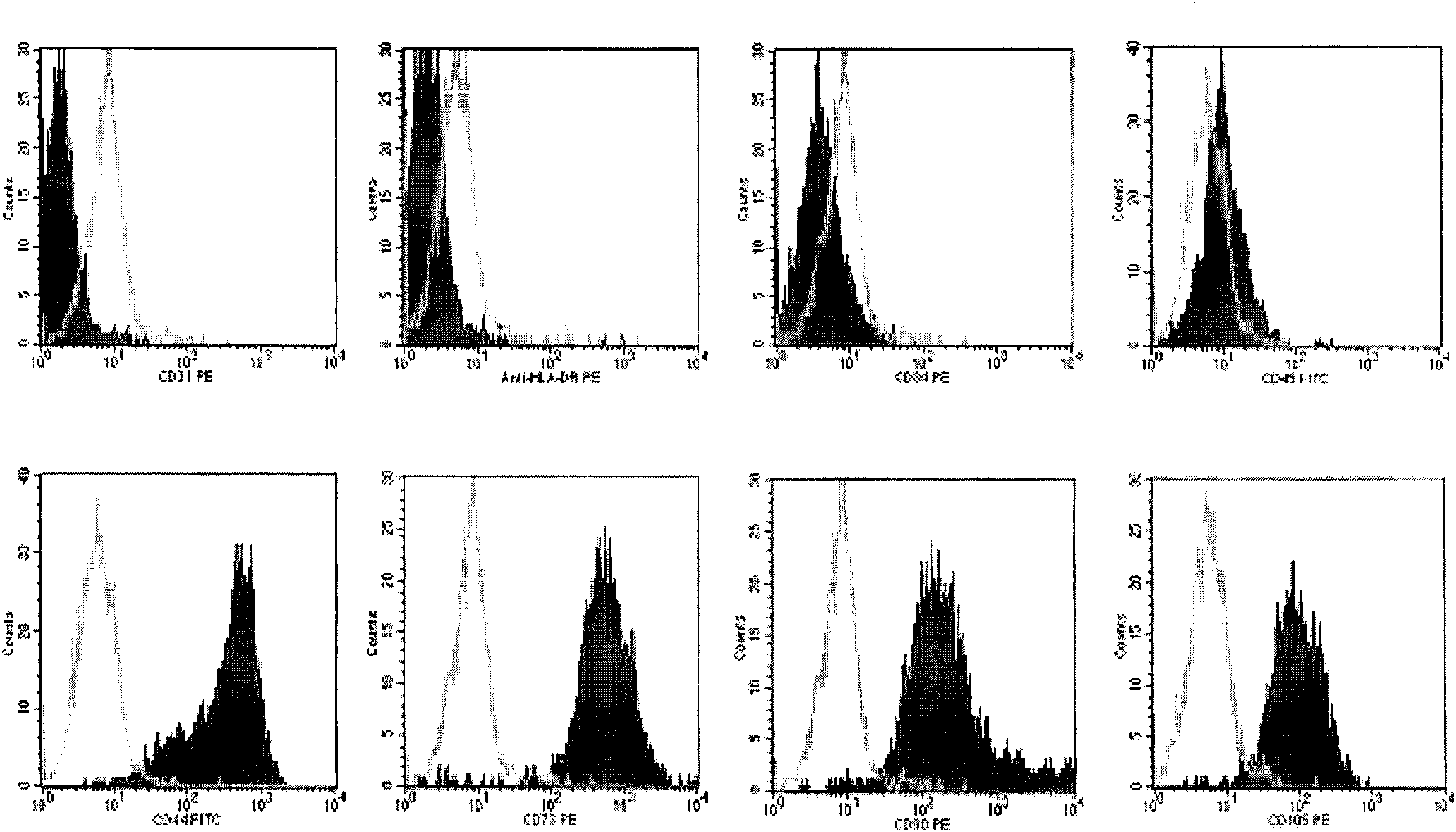
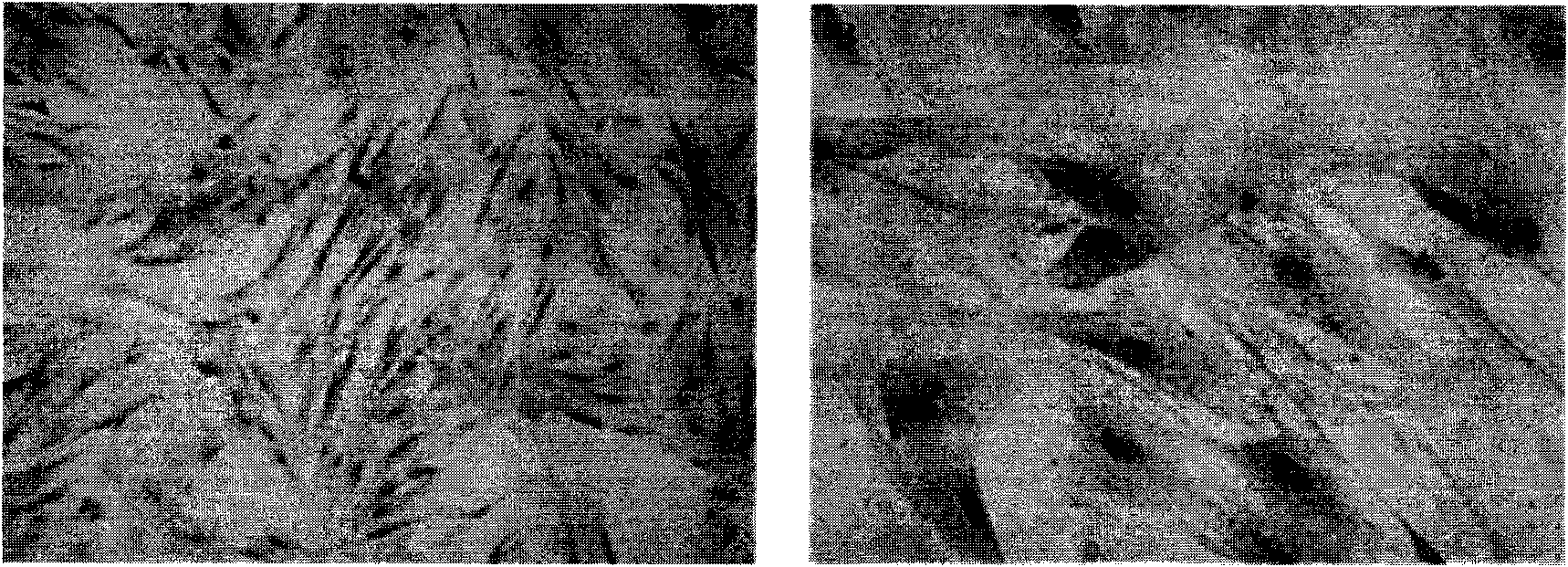Preparation and storage of umbilical cord-mesenchymal stem cells (UC-MSCs) used in clinical therapy
A stem cell and umbilical cord technology, which is applied in the field of preparation and storage of human umbilical cord mesenchymal stem cells, can solve the problems of increasing the safety risk of clinical application, existing allergies and cross-infection, and low purity of primary cultured cells, and achieves good application prospects. , excellent effect, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Under aseptic conditions, take the umbilical cord of the normal delivery fetus, the umbilical cord is about 20cm long, and wash it repeatedly with normal saline containing 2% gentamicin to remove the residual blood. At the same time, the pathogenic microorganism detection is carried out, and the following steps are carried out after being completely qualified.
[0022] 2. Place the umbilical cord in physiological saline containing 2% gentamicin, cut it into particles with sterile scissors, collect the tissue pieces and place them in a 50ml centrifuge tube, and centrifuge at a high speed at 2000 rpm , centrifuged for 10 minutes, discarded the supernatant, re-added physiological saline containing 2% gentamicin, fully oscillated the precipitated tissue pieces to make them loose, and centrifuged at a high speed at 2000 rpm for 10 minutes. .
[0023] 3. Suspend the tissue block obtained in step 2 in DMEM medium containing 10% fetal bovine serum, shake fully and centrifug...
Embodiment 2
[0031] 1. Under aseptic conditions, take the umbilical cord of the fetus delivered by cesarean section, the umbilical cord is about 25cm long, and wash it repeatedly with physiological saline containing 2% gentamicin to remove residual blood. At the same time, the pathogenic microorganism detection is carried out, and the following steps are carried out after being completely qualified.
[0032] 2. Place the umbilical cord in physiological saline containing 2% gentamicin, cut it into particles with sterile scissors, collect the tissue pieces and place them in a 50ml centrifuge tube, and centrifuge at a high speed at 2000 rpm , centrifuged for 10 minutes, discarded the supernatant, re-added physiological saline containing 2% gentamicin, fully oscillated the precipitated tissue pieces to make them loose, and centrifuged at a high speed at 2000 rpm for 10 minutes. .
[0033] 3. Suspend the tissue block obtained in step 2 in DMEM medium containing 10% fetal bovine serum, shake fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com