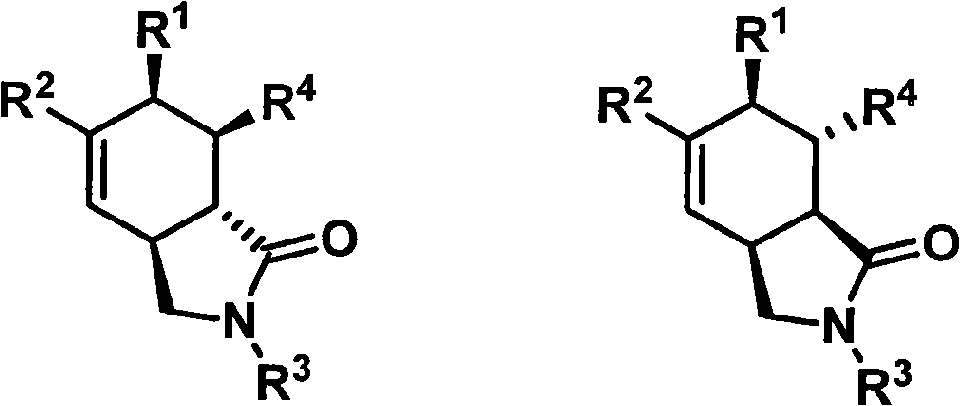
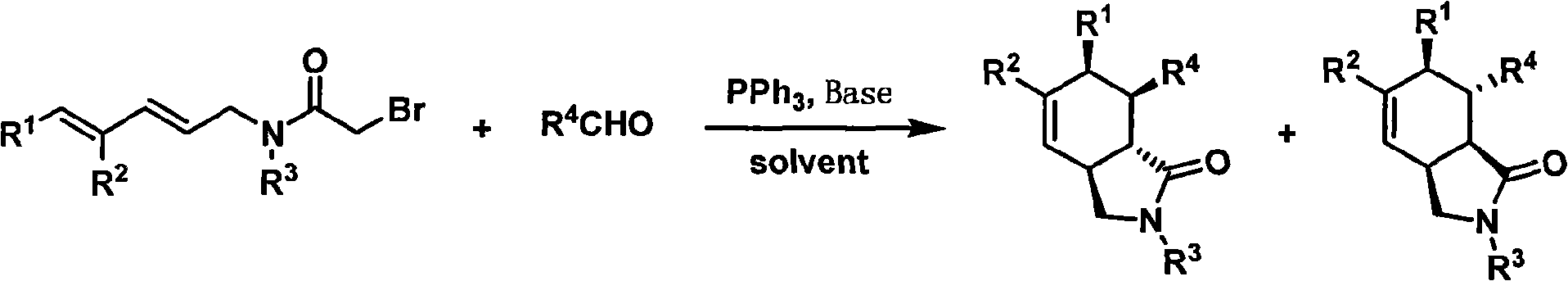
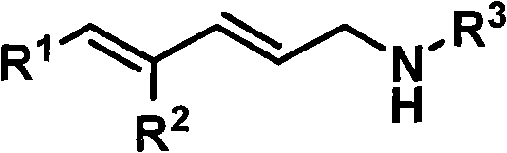
Hexahydroisoindole-1-keto compound and synthetic method thereof
A technology for hexahydroisoindole and ketone compounds, which is applied in organic chemistry and other fields, and can solve problems such as the limitation of synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (3aS * , 6R * , 7R * , 7aR * Synthesis of )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6-phenyl-7-benzoyl-1H-isoindol-1-one Proceed as follows:
[0052] (2E, 4E)-N-(4-methoxybenzyl)-5-phenylpenta-2,4-dienyl α-bromoacetamide (114 mg, 0.28 mmol) in THF-water mixed solution (H 2 (0:THF=1:10, 3ml) was added triphenylphosphine (88 mg, 0.34mmol), potassium carbonate (58 mg, 0.42mmol) and hydrated acetophenone aldehyde (51 mg, 0.34mmol), and the mixture The reaction was stirred at room temperature for 6 hours. The reaction mixture was diluted with 10 mL of ethyl acetate, washed successively with saturated aqueous ammonium chloride solution and saturated brine (5 mL each), and the combined aqueous phase was extracted with ethyl acetate (3×5 mL). After combining the organic phases with anhydrous Na 2 SO 4 Dry, filter, and remove the solvent under reduced pressure to obtain a crude product, which is purified by silica gel column chromatography with a petroleum ether sol...
Embodiment 2
[0054] (3aS * , 6R * , 7R * , 7aR * Synthesis of )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6-phenyl-7-ethoxycarbonyl-1H-isoindol-1-one
[0055] Mixed solution of (2E, 4E)-N-(4-methoxybenzyl)-5-phenylpenta-2,4-dienyl α-bromoacetamide (60 mg, 0.15 mmol) in tetrahydrofuran-water (H 2(0:THF=2:1, 2ml) was added triphenylphosphine (59mg, 0.22mmol), potassium carbonate (22mg, 0.16mmol) and ethyl glyoxylate (46mg, 0.45mmol), and the mixture The reaction was stirred at room temperature for 48 hours. Carry out aftertreatment by the method for embodiment 1, obtain product (3aS * , 6R * , 7R * , 7aR * )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6-phenyl-7-ethoxycarbonyl-1H-isoindol-1-one 29 mg , the yield was 48%. colorless oil; 1 H NMR (400MHz, CDCl 3 )δ7.32-7.21(m, 3H), 7.20-7.12(m, 4H), 6.86(d, J=8.4Hz, 2H), 6.02(d, J=9.6Hz, 1H), 5.71(ddd, J =9.6, 3.2, 3.2Hz, 1H), 4.49 and 4.25(ABq, J=14.4Hz, 2H), 4.02-3.96(m, 1H), 3.80(s, 3H), 3.78-3.64(m, 2H), 3.31(dd, J=9.2, 7.2...
Embodiment 3
[0057] (3aS * , 6R * , 7R * ,7aS * )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6,7-diphenyl-1H-isoindol-1-one and (3aS * , 6R * ,7S * , 7aR * Synthesis of )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6,7-diphenyl-1H-isoindol-1-one
[0058] Will contain (2E, 4E)-N-(4-methoxybenzyl)-5-phenylpenta-2,4-dienyl α-bromoacetamide (32 mg, 0.08 mmol,), triphenyl Phosphine (26 mg, 0.10mmol), potassium carbonate (17 mg, 0.12mmol) and benzaldehyde (17 mg, 0.16mmol) in tetrahydrofuran-water mixed solution (H 2 (0:THF=1:1, 1 ml) microwave reaction tube was capped and sealed, stirred and reacted at room temperature for 15 hours, and then heated by microwave at 180° C. for 0.5 hour. Carry out aftertreatment by the method for embodiment 1, obtain product (3aS * , 6R * , 7R * ,7aS * )-2,3,3a,6,7,7a-hexahydro-2-(4-methoxybenzyl)-6,7-diphenyl-1H-isoindol-1-one 23 mg, yield 70%. colorless oil; 1 H NMR (400MHz, CDCl 3 )δ7.17(d, J=8.4Hz, 2H), 7.10-7.03(m, 6H), 6.87(d, J=8.8Hz, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com