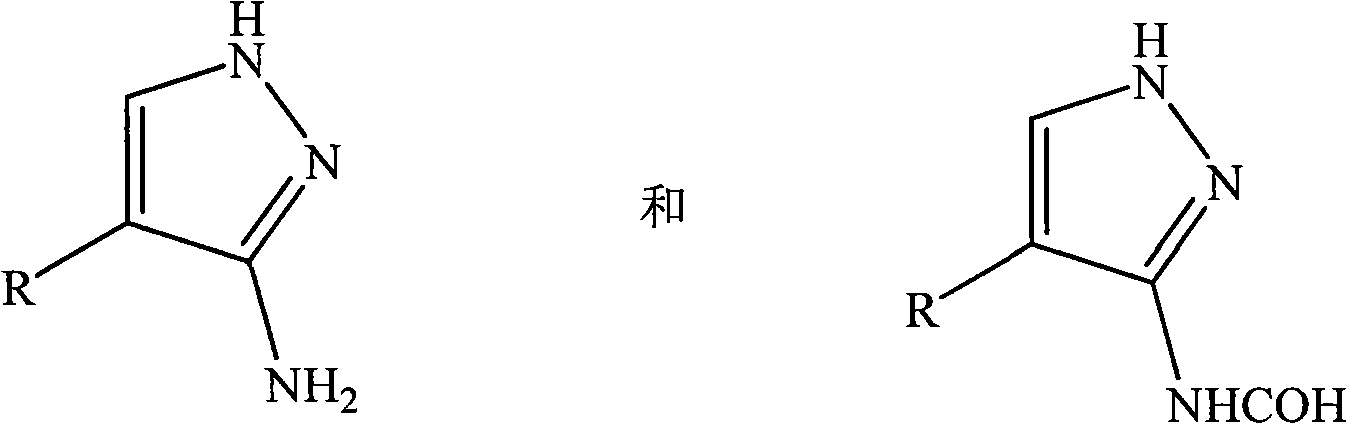
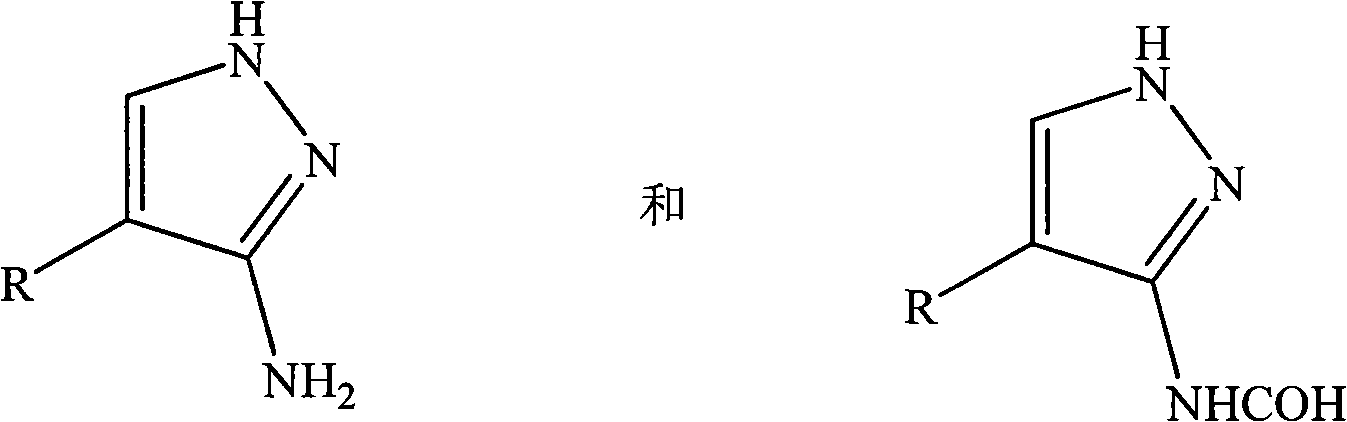
Method for preparing 3-N-formylated aminopyrazole compound
A technology for formylating aminopyrazole and aminopyrazole is applied in the field of preparation of 3-N-formylated aminopyrazole compounds, and can solve the difficulty of extracting and purifying 3-N-formylated aminopyrazole compounds, There are no problems such as physical and chemical properties, and even the impact of clinical applications, to achieve the effects of high yield, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] A preparation method of 3-N-formylated aminopyrazole compound, the preparation method comprising the steps of:
[0014] (1) In a reaction vessel, a 3-aminopyrazole compound is dissolved in a mixed solution of an acylating agent and formic acid. Wherein, the volume ratio of the acylating agent to formic acid is 60:1, and according to the concentration of the 3-aminopyrazole compound, the mixed solution of the acylating agent and formic acid is configured in excess to facilitate the complete conversion of the 3-aminopyrazole compound. Then, fully stir under the action of microwave, and heat and keep warm for 40min. Wherein, the microwave power is 900W, and the reaction temperature is 100°C.
[0015] (2) After the reaction is completed, cool to room temperature, and then remove the unreacted acylating agent and formic acid mixed solution by suction filtration to obtain a crude product containing 3-N-formylated aminopyrazole compound.
[0016] (3) Rinse the crude product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com