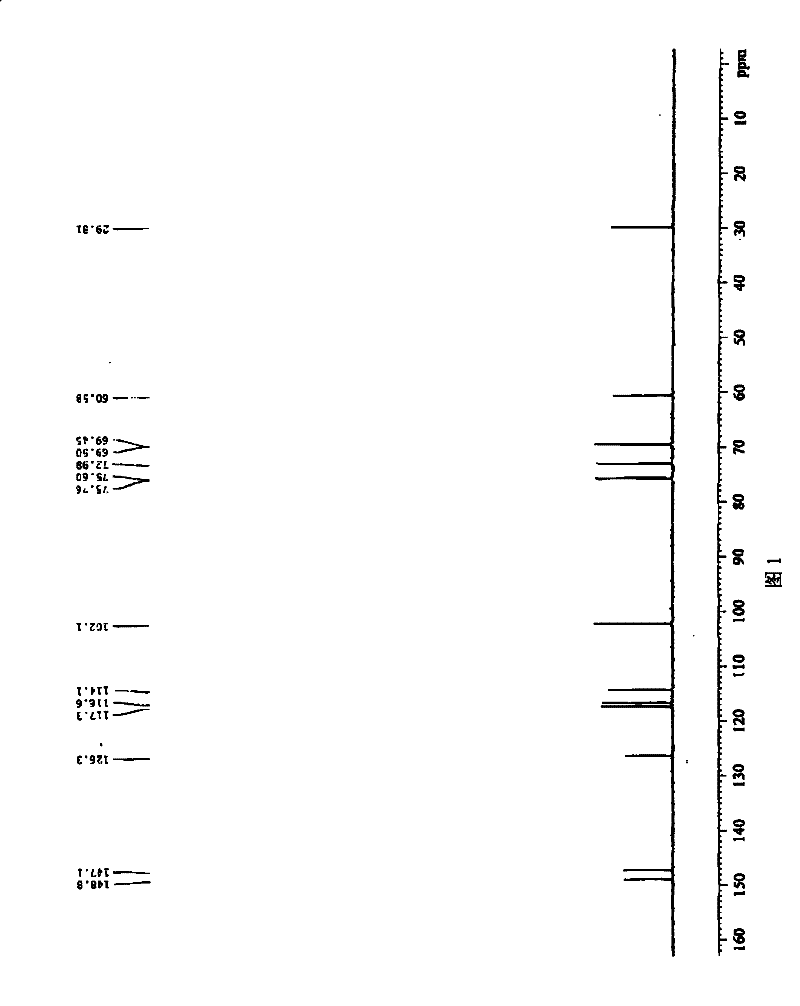
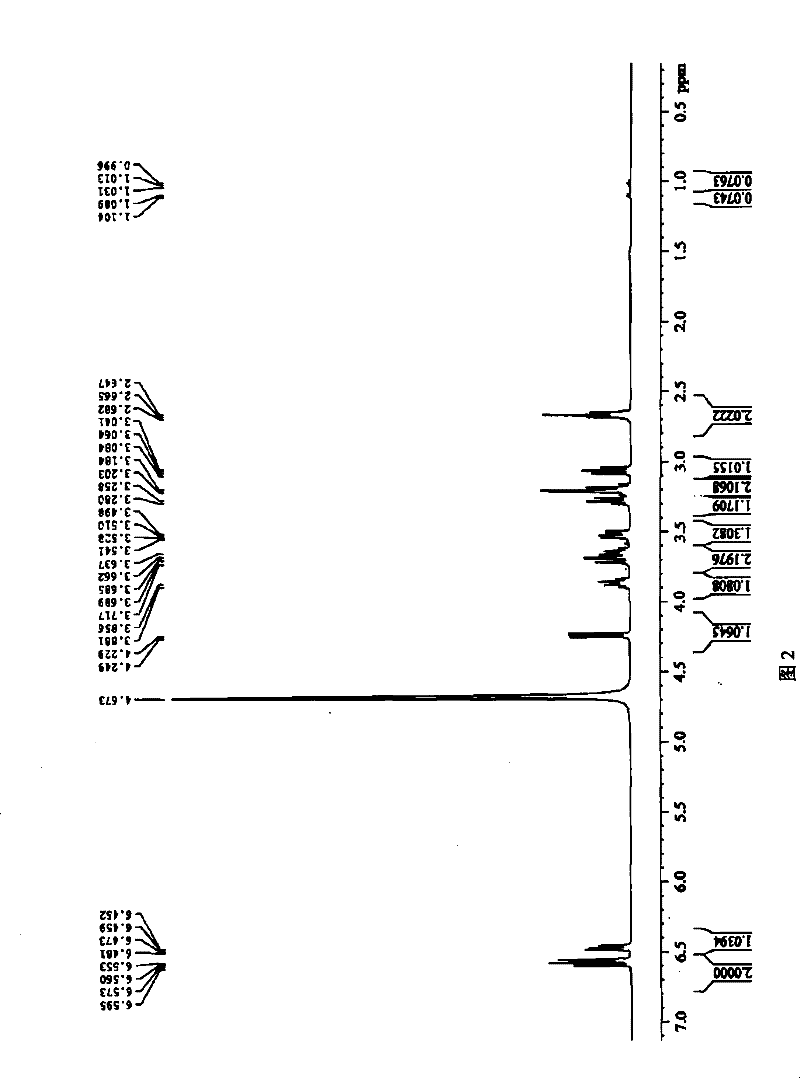
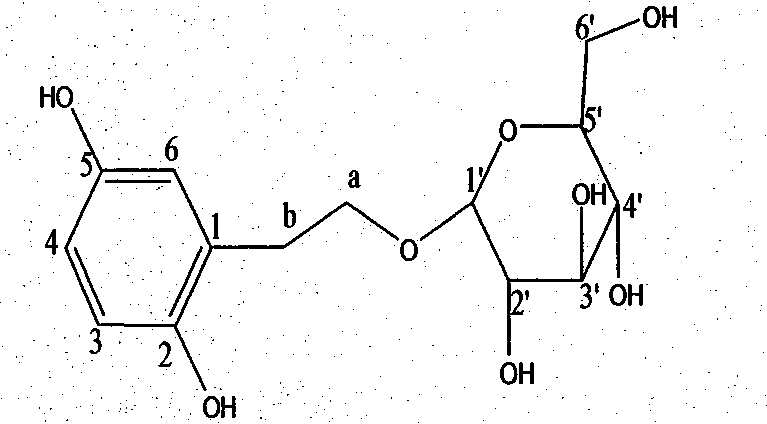
A kind of dihydroxyphenylethanol glucoside compound and its preparation method
A technology of dihydroxyphenylethanol and glucoside, which is applied in the field of phenylethanol glycosides to achieve the effects of good reproducibility, stable preparation performance and high sample purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Forsythia raw medicinal material was crushed, weighed 1 kg, placed in a 50 liter extraction tank, decocted with 10 liters of water for 2 hours, filtered, and the filtrate was stored for later use. Add 10 liters of water to the filter residue and decocted for 2 hours, filtered, and the filtrate Combine this time and discard the filter residue. Rotary evaporation of the filtrate to obtain 190ml of extract (relative density 1.05). The extract was dissolved in an equal volume of water, added with 653 ml of 95% ethanol, stirred, allowed to stand for 24 hours, filtered, and the filter residue was discarded. The filtrate was concentrated until no alcohol smell, and 142 ml of forsythia alcohol precipitation fraction was obtained.
[0022] The forsythia alcohol precipitation component was made up to 200 ml with water, and 28.4 g of sodium bicarbonate was added, stirred, reacted for 3 hours, and left overnight. Gradually add 56.8 ml of 1:1 hydrochloric acid and 1.4 ml of 1:2 hydroc...
Embodiment 2
[0036] Forsythia raw medicinal materials were crushed, weighed 3 kg, placed in a 100 liter extraction tank, decocted with 30 liters of water for 2 hours, filtered, and the filtrate was stored for later use. Add 30 liters of water to the filter residue and decocted for 2 hours. Combine this time and discard the filter residue. Rotary evaporation of the filtrate to obtain 570ml of extract (relative density 1.05). The extract was dissolved in an equal volume of water, added with 1959 ml of 95% ethanol, stirred, allowed to stand for 24 hours, filtered, and the filter residue was discarded. The filtrate was concentrated until there was no alcohol smell to obtain 426 ml of forsythia alcohol precipitation fraction.
[0037] The forsythia alcohol precipitation component was made up to 600 ml with water, 85.2 g of sodium bicarbonate was added, stirred, reacted for 3 hours, and left overnight. Gradually add 170.4 ml of 1:1 hydrochloric acid and 4.1 ml of 1:2 hydrochloric acid (the pH is a...
Embodiment 3
[0042] Forsythia raw medicinal materials were crushed, weighed 6.8 kg, placed in a 100 liter extraction tank, decocted with 68 liters of water for 2 hours, filtered, and the filtrate was stored for later use. Add 68 liters of water to the filter residue and decocted for 2 hours. Combine this time and discard the filter residue. The filtrate was rotary evaporated to obtain 1292ml of extract (relative density 1.05). The extract was dissolved in an equal volume of water, added 4.44 liters of 95% ethanol, stirred, allowed to stand for 24 hours, filtered, and the filter residue was discarded. The filtrate was concentrated until there was no alcohol smell to obtain 966 ml of forsythia alcohol precipitation.
[0043] The forsythia alcohol precipitation component was made up to 1.36 liters with water, and 193 g of sodium bicarbonate was added, stirred, reacted for 3 hours, and left overnight. Gradually add 386 ml of 1:1 hydrochloric acid and 9.3 ml of 1:2 hydrochloric acid (the pH is ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com