Rat bone marrow tumour cell NSO and preparation method and application thereof
A technology for myeloma cells and mouse myeloma, which is applied in the field of mouse myeloma cell NSO and its preparation, can solve the problems of increasing purification costs, increasing the probability of infection, and purification difficulty, so as to reduce purification costs and losses, and avoid animal-derived components and unsafe factors, and the effect of reducing the cost of cultivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, prepare the mouse myeloma cell NSO that can grow in serum-free, lipid-free medium
[0047] 1. Preparation
[0048] Mouse myeloma cells NSO cells were purchased from ECACC (The European Collection of Animal Cell Culture).
[0049] (1) Cells were placed in cell culture medium I, and cultured at 37°C for 3 days; the composition of cell culture medium I was: RPMI1640 medium containing 10% (volume percentage) fetal bovine serum (Table 1), The pH is 7.4.
[0050] (2) Carry out a subculture of cells: cells are placed in cell culture medium II, and cultivated at 37° C. for 3 days; Culture medium I), adding an equal volume of hybridoma serum-free medium (Invitrogen Company Catalog Number: 11279-023) containing 1% (volume percentage) bovine serum albumin (BSA) with a pH of 7.4 to allow cell culture The final concentration of fetal bovine serum in Base II was reduced to 5%.
[0051] (3) Carry out secondary cell subculture: cells are placed in cell culture medium ...
Embodiment 2
[0062] Example 2. Production of Human TNF-alpha Soluble Receptor Using Mouse Myeloma Cell NSO-Ch1 CGMCC No.2127
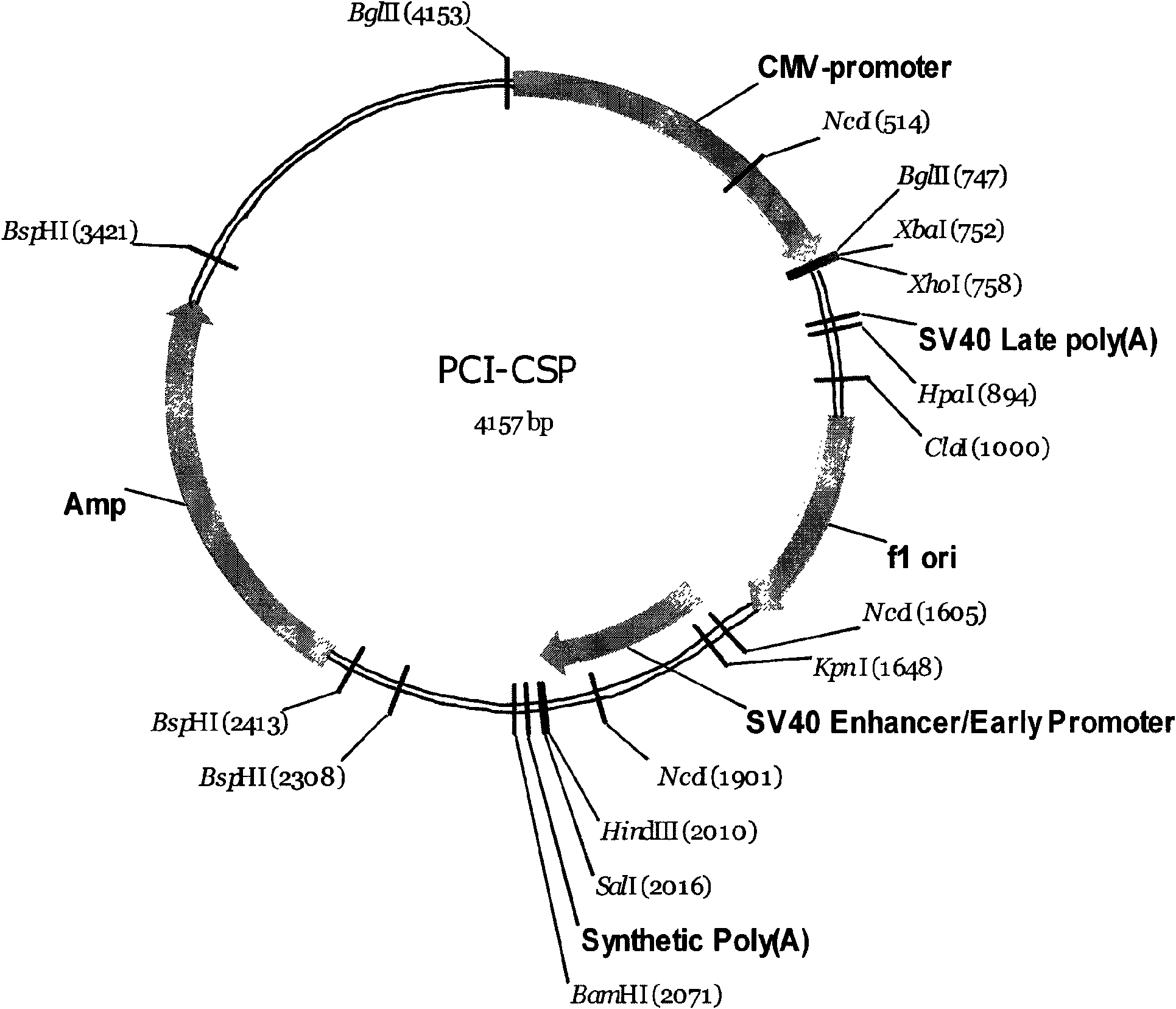
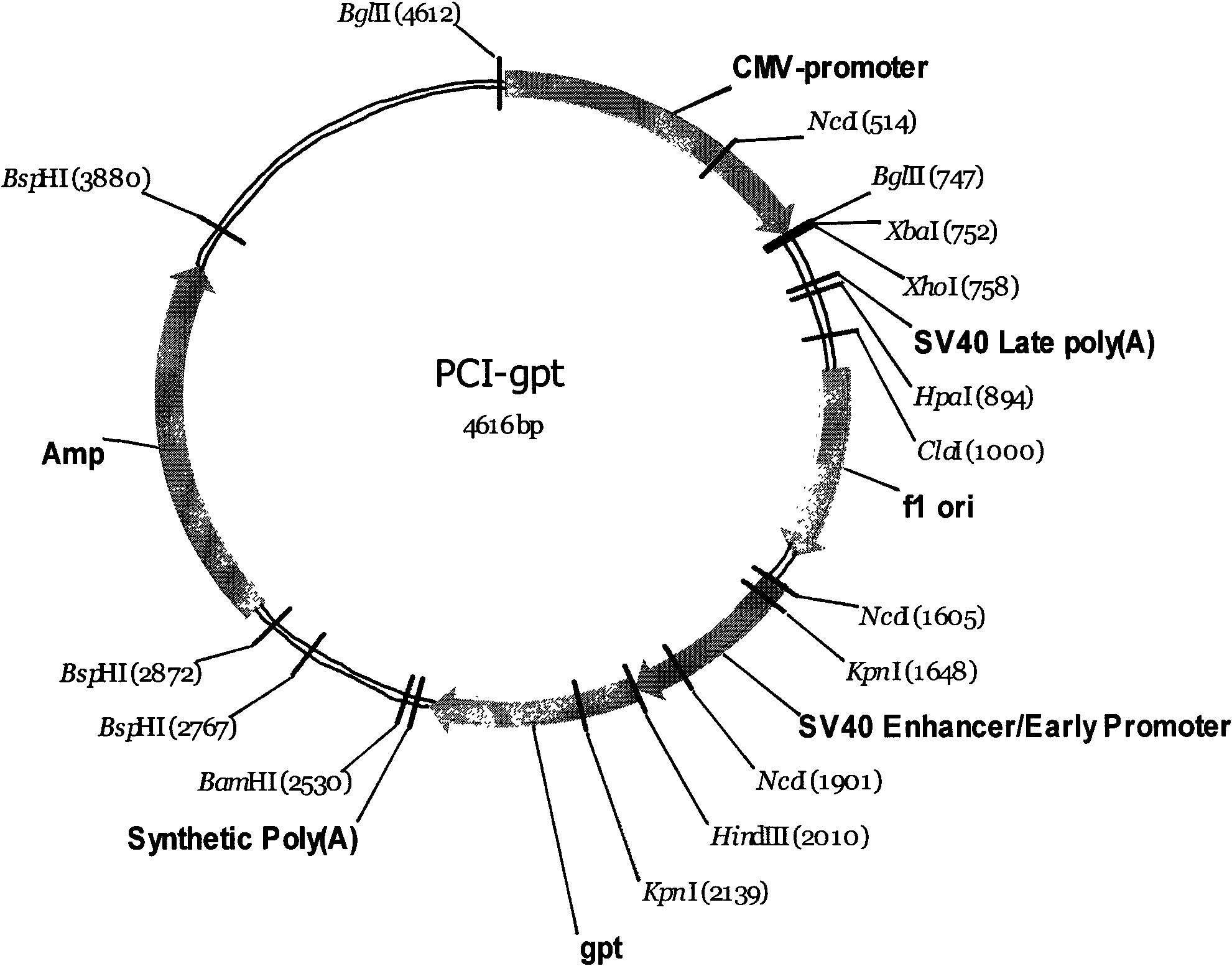
[0063] The cDNA sequence encoding the extracellular segment of the human TNF-alpha type II receptor and the cDNA sequence encoding the Fc segment of human immunoglobulin IgG1 were constructed by PCR into a recombinant gene (TNFR-Fc) (shown in sequence 1 in the sequence listing); TNFR-Fc is inserted into the Xba1 and Xho1 sites of the expression vector pCI-gpt with a selectable marker (guanine phosphoribosyltransferase, gpt) and a gene expression regulatory region (CMV promoter, terminator), to obtain the expression of the recombinant protein Vector pCI-gpt-TNFR-Fc. Linearize pCI-gpt-TNFR-Fc with restriction endonuclease FspI, and introduce it into NSO-Ch1 (CGMCC No.2127) cells by electroporation. The transfected cells were screened with Mycophenolate in medium containing Xanthine to obtain stably transfected cell lines.
[0064] The successfully transfected cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com