Application of medicine combination in preparing preparation for treating chronic hepatitis B
A technology for chronic hepatitis B and a composition is applied in the application field of preparing preparations for treating chronic hepatitis B, and can solve the problems of repeated disease, HBV mutation, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
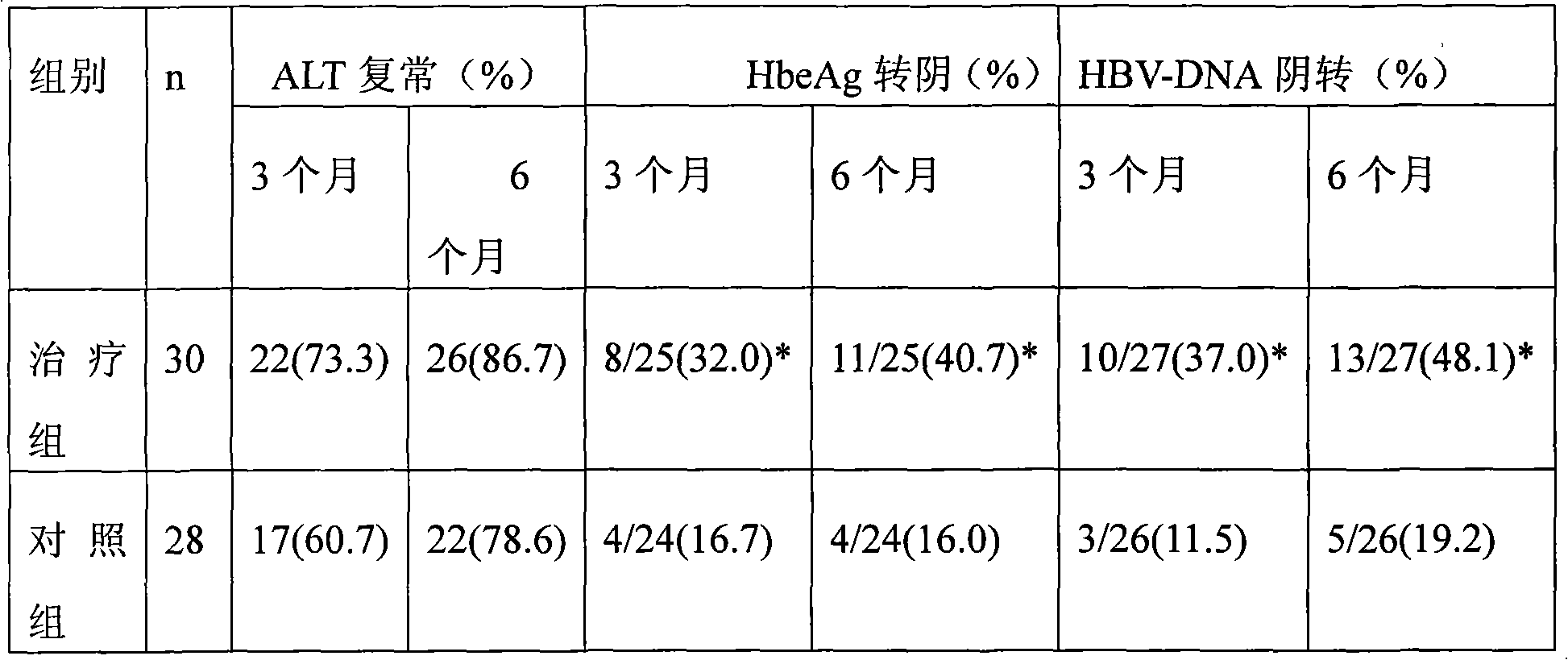
Image
Examples
Embodiment 1
[0020] The BCG polysaccharide nucleic acid required by the present invention can be obtained by the following methods, but those skilled in the art can understand that the preparation of BCG polysaccharide nucleic acid includes but is not limited to this method:
[0021] 1) Cultivation and harvest of BCG
[0022] 1. Bacterial culture: Dissolve the strains preserved in liquid cryogenic temperature (BCG strain D2PB302 for the preparation of BCG in China, China National Institute for the Control of Pharmaceutical and Biological Products) at room temperature, inoculate in potato Sutong culture medium, and culture continuously at 37°C for 14-20 days ; or after continuous cultivation at 37°C for 15 days, transfer to improved liquid Sutong medium, and continuous cultivation at 37°C for 14-20 days.
[0023] 2. Bacteria collection: When the bacteria grow to the logarithmic phase, check the culture bottles one by one, collect the bacteria film, add an appropriate amount of deionized dis...
Embodiment 2
[0040] A kind of production method of the pharmaceutical composition injection for the treatment of chronic hepatitis B, prescription is:
[0041] BCG nucleic acid: 0.3g
[0042] Physiological sodium chloride solution (0.9%): 100ml
[0043] Take the ordinary freeze-dried powder D BCG polysaccharide nucleic acid of prescription quantity and mix uniformly with appropriate amount of water for injection, and those skilled in the art can also understand that the BCG polysaccharide nucleic acid can be obtained in the form of micropowder, solid dispersion, clathrate, microsphere, Add in the form of liposomes or microparticles. After fully dissolving, centrifuge at a speed of 4000 rpm and 3°C for 15 minutes. Take the supernatant and test the content of polysaccharides and nucleic acids. After the test is qualified, the supernatant passes through 0.22 Finely filter with a μm filter, then fill and seal in an ampoule, and sterilize by high-pressure steam to obtain the injection.
Embodiment 3
[0045] A kind of production method of the pharmaceutical composition powder injection for treating chronic hepatitis B, prescription is:
[0046] BCG nucleic acid: 0.3g
[0047] Physiological sodium chloride solution (0.9%): 100ml
[0048]Take the ordinary freeze-dried powder D BCG polysaccharide nucleic acid of prescription quantity and mix uniformly with appropriate amount of water for injection, and those skilled in the art can also understand that the BCG polysaccharide nucleic acid can be obtained in the form of micropowder, solid dispersion, clathrate, microsphere, Add in the form of liposomes or microparticles. After fully dissolving, centrifuge at a speed of 4000 rpm and 3°C for 15 minutes. Take the supernatant and test the content of polysaccharides and nucleic acids. After the test is qualified, the supernatant passes through 0.22 The μm filter is used for fine filtration, and the filtrate is sterilized by high-pressure steam, then packed into vials, freeze-dried, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com