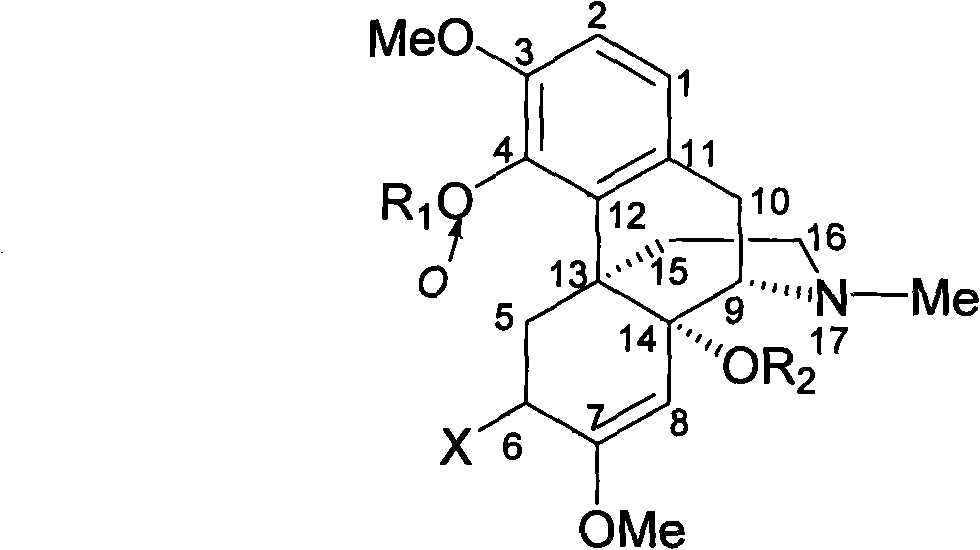
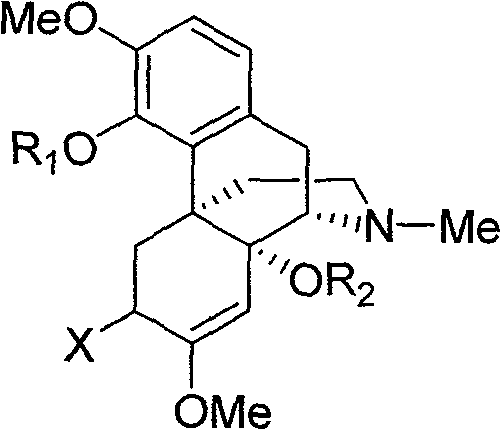
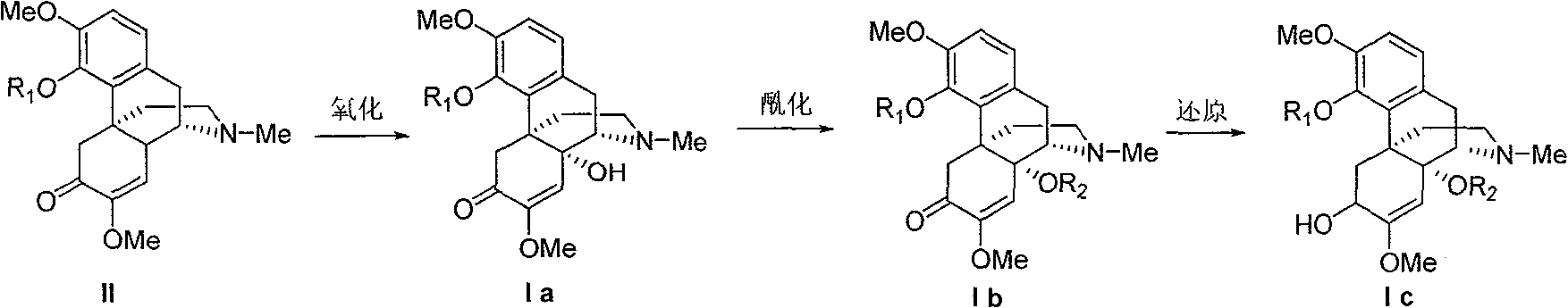
14-hydroxyl sinomenine derivatives, synthesis method thereof and use thereof
A technology of hydroxysinomenine and derivatives, applied in the field of compounds, can solve the problems of slow onset, low bioavailability, weak action intensity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 14-Hydroxy-O-methylsinomenine (Compound 1)
[0042] 0.5g O-methyl sinomenine was dissolved in 30mL dichloromethane, and 5g active MnO was added in batches 2 , warming up to reflux for 42h. Cool to room temperature, filter, and concentrate the filtrate to obtain a crude product, which is separated by silica gel column chromatography (eluent: ethyl acetate) to obtain compound 1, yield: 13%, mp: 157.3-157.5°C.
Embodiment 2
[0044] 6,14-Dihydroxy-O-methyl sinomenine (Compound 2)
[0045] Dissolve 0.3g of 14-hydroxy-O-methylsinomenine in 10mL of anhydrous methanol, cool to 0°C in an ice-water bath, add 0.3g of NaBH 4 , warmed to room temperature and stirred for 2h, concentrated the reactant, and added 10mL of water and 0.3g of NH to the obtained solid 4 Cl, stirred at room temperature for 15 min, extracted with 2×20 mL of dichloromethane, combined organic layers, and dried over anhydrous sodium sulfate. The residue after removing the solvent under reduced pressure was separated by silica gel column chromatography (eluent: ethyl acetate) to obtain compound 2, yield: 88%, mp: 167.2-169.0°C.
Embodiment 3
[0047] 14-Acetoxy-O-methyl sinomenine (Compound 3)
[0048] Mix 0.5g 14-hydroxy-O-methyl sinomenine, 20mL dichloromethane and 1mL acetic anhydride, start to stir, reflux for 2h, cool, spin off the solvent under reduced pressure, pack the column with silica gel chromatography, and then wash with ethyl acetate , and 20:1 chloroform / methanol eluting to obtain compound 3, yield: 78%, mp: 149.5-152.1°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com