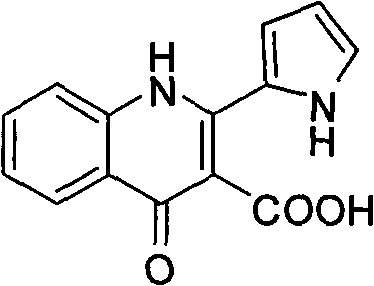
Quinolinone alkaloid derivant as well as preparation method and application thereof
A technology of alkaloid derivatives and quinolinones is applied in the application field of microbial pesticides and insecticides, which can solve problems such as insecticides that have not yet been seen, and achieve the effect of broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0009] In preparing the compounds of the present invention, the following steps are taken:
[0010] (1) Carry out strain culture to marine fungus (Penicillium sp.ZZF32#) in strain culture medium earlier, the culture medium used contains glucose 1.0% (percentage by weight, the same below), yeast extract 0.1%, peptone 0.2%, Agar 1.0%, sodium chloride 0.3%, the rest is water. When used, it was made into a test tube slant, and the above-mentioned fungal strains were cultured at 30° C. for 5 days.
[0011] The strain medium contains 0.1%-5.0% of glucose, 0.01%-1% of yeast extract, 0.01%-1% of peptone, 0.1%-3.0% of agar, 0.05%-5% of sodium chloride and the rest is water. The culture temperature is 15-35° C., and the culture time is 3-10 days.
[0012] (2) The fungal strain obtained from the above culture is fermented and cultured, and the used fermentation medium contains 1.0% of glucose, 0.1% of yeast extract, 0.2% of peptone, 0.3% of sodium chloride, and the rest is water. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com