Method for recrystallizing and refining fluticasone propionate
A fluticasone propionate and recrystallization technology, which is applied in the fields of steroids, respiratory diseases, organic chemistry, etc., can solve the problems of fluticasone propionate impurities, etc., and achieve the effects of guaranteed yield, easy industrialization, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
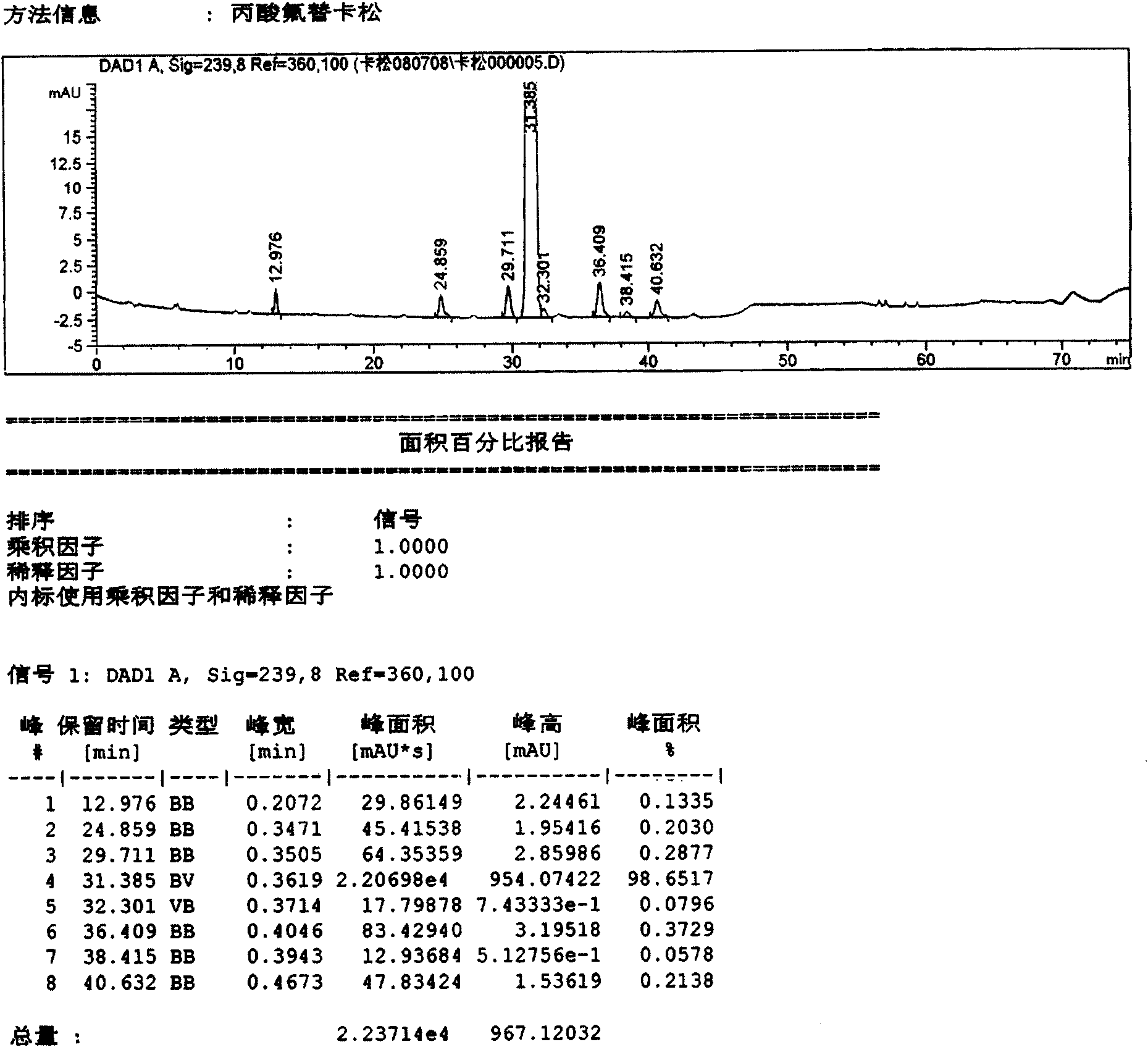
[0038]Using conventional methods, use bromofluoromethane with 6α,9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrost-1,4-diene-17β-thiocarboxylic acid Reaction obtains fluticasone propionate crude product (sample 1), records liquid phase diagram, sees attached figure 1 . It can be seen from the figure that the main content is: 98.617%; impurities C, D, F, and G are 0.2030%, 0.2877%, 0.3729%, and 0.2138% respectively. In addition, A, B, E, H, I impurities and other unknown impurities are not more than 0.2%
Embodiment 2
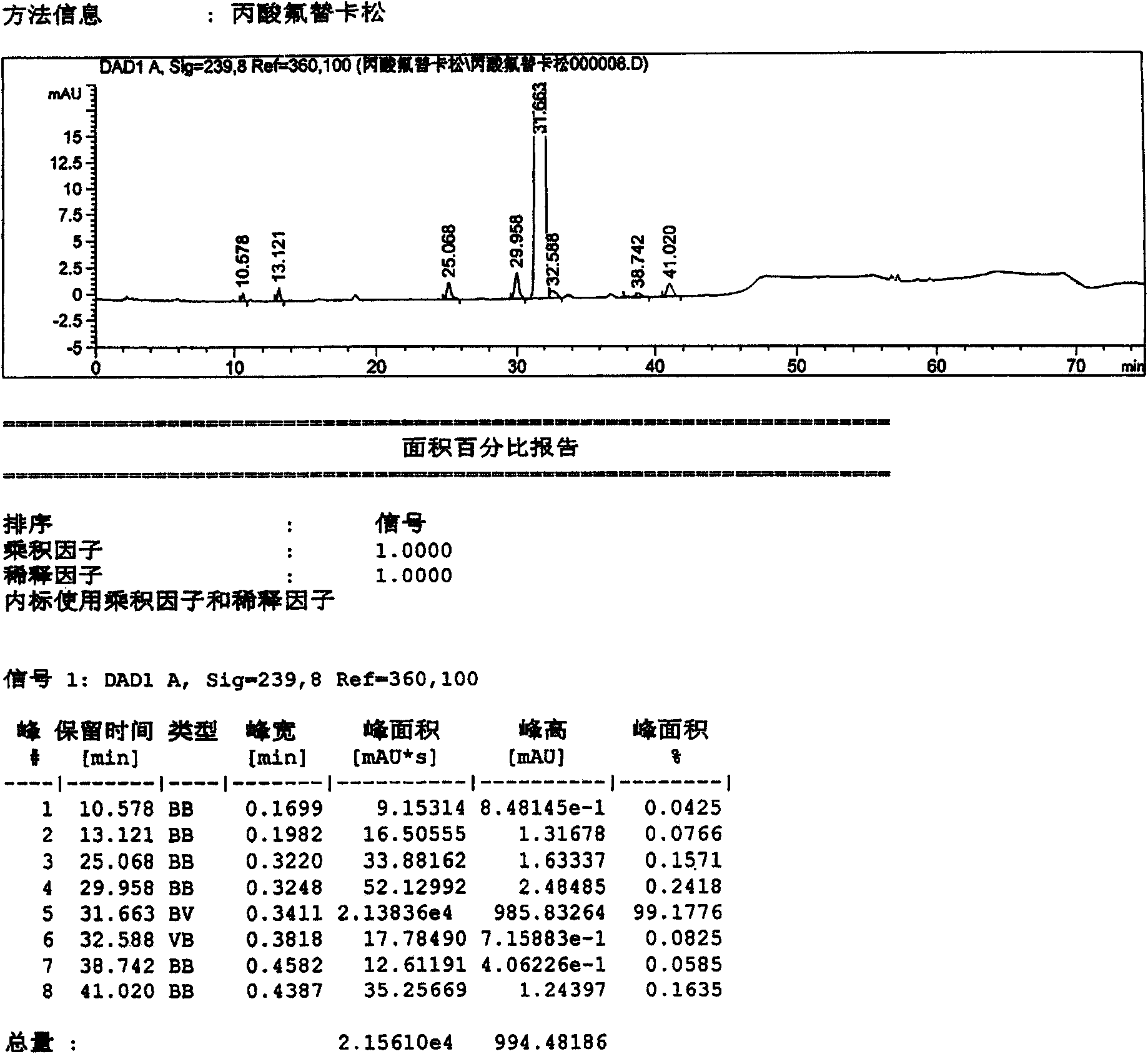
[0040] Dissolve 10 g of the crude fluticasone propionate obtained in Example 1 in 100 ml of dioxane solution, concentrate under reduced pressure at 65°C, and concentrate until the volume of recrystallization is about 30ml, slowly cool down, and keep warm at 5°C for crystallization 12 hours, filter, obtain 9.03g fluticasone propionate refined product (sample 2), record liquid phase figure, see attached figure 2 . According to the relative retention time, it can be seen that the main content is: 99.1776%; impurities C, D, and G are 0.1571%, 0.2418%, and 0.1635%, respectively. The remaining unknown impurities are all below 0.15%.
Embodiment 3
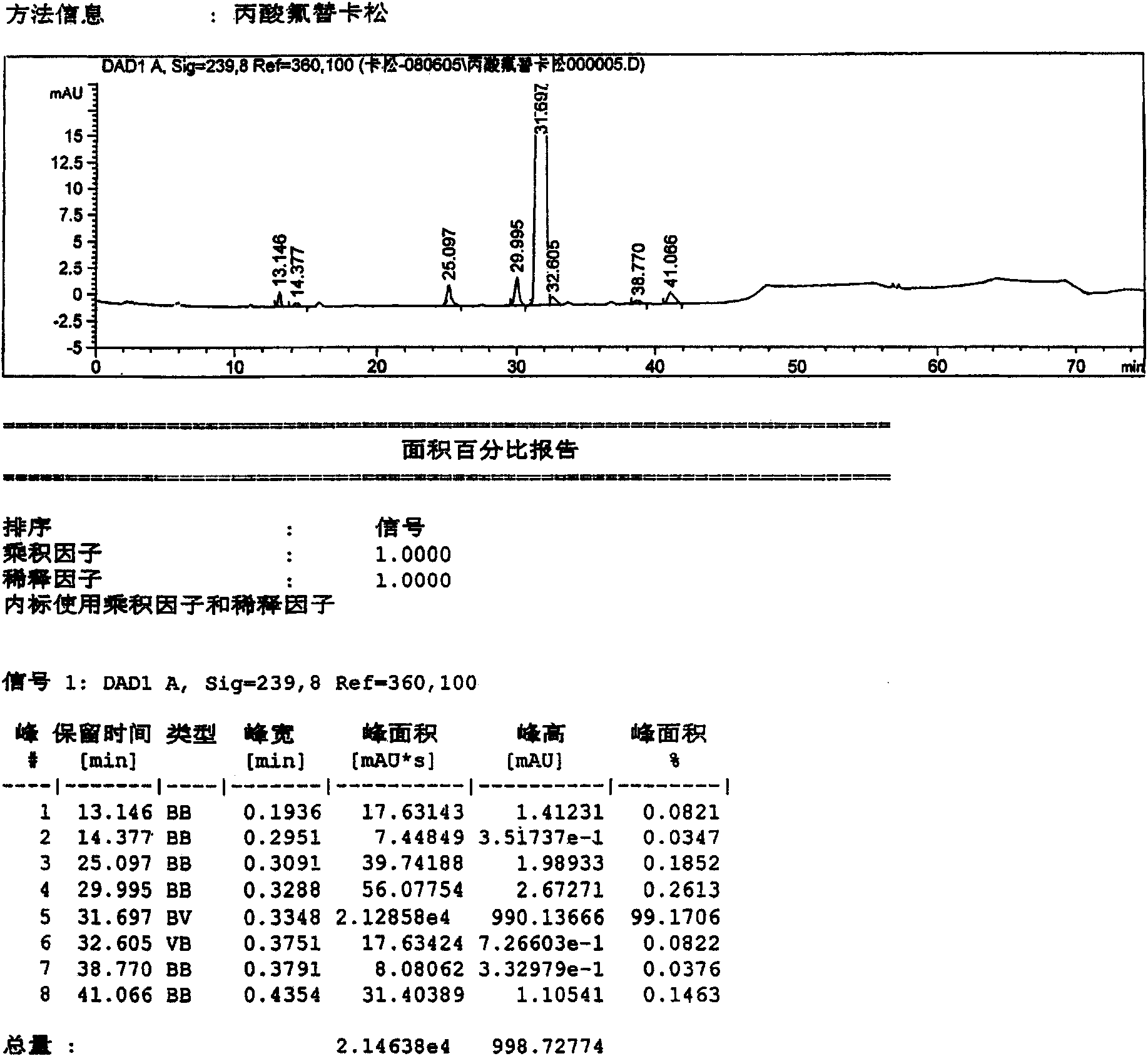
[0042] Dissolve 10 g of the crude fluticasone propionate obtained in Example 1 in 80 ml of tetrahydrofuran solution, concentrate under reduced pressure at 55°C, and concentrate until the volume of recrystallization is about 25ml, add 1ml of water dropwise, slowly cool down, and Insulated and crystallized for 12 hours, filtered to obtain 9.12g fluticasone propionate elaboration (sample 3), recorded liquid phase diagram, see attached image 3 . According to the relative retention time, it can be seen that the main content is: 99.1706%; impurities C, D, and G are 0.1852%, 0.2613%, and 0.1463%, respectively. The remaining unknown impurities are all below 0.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com