Biomarker of Parkinson disease
A technology for biomarkers and Parkinson's disease, applied in the direction of biological testing, microbiological determination/examination, biochemical equipment and methods, etc., can solve problems such as complex diagnosis, difficult diagnosis of Parkinson's disease, lack of clinical symptoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
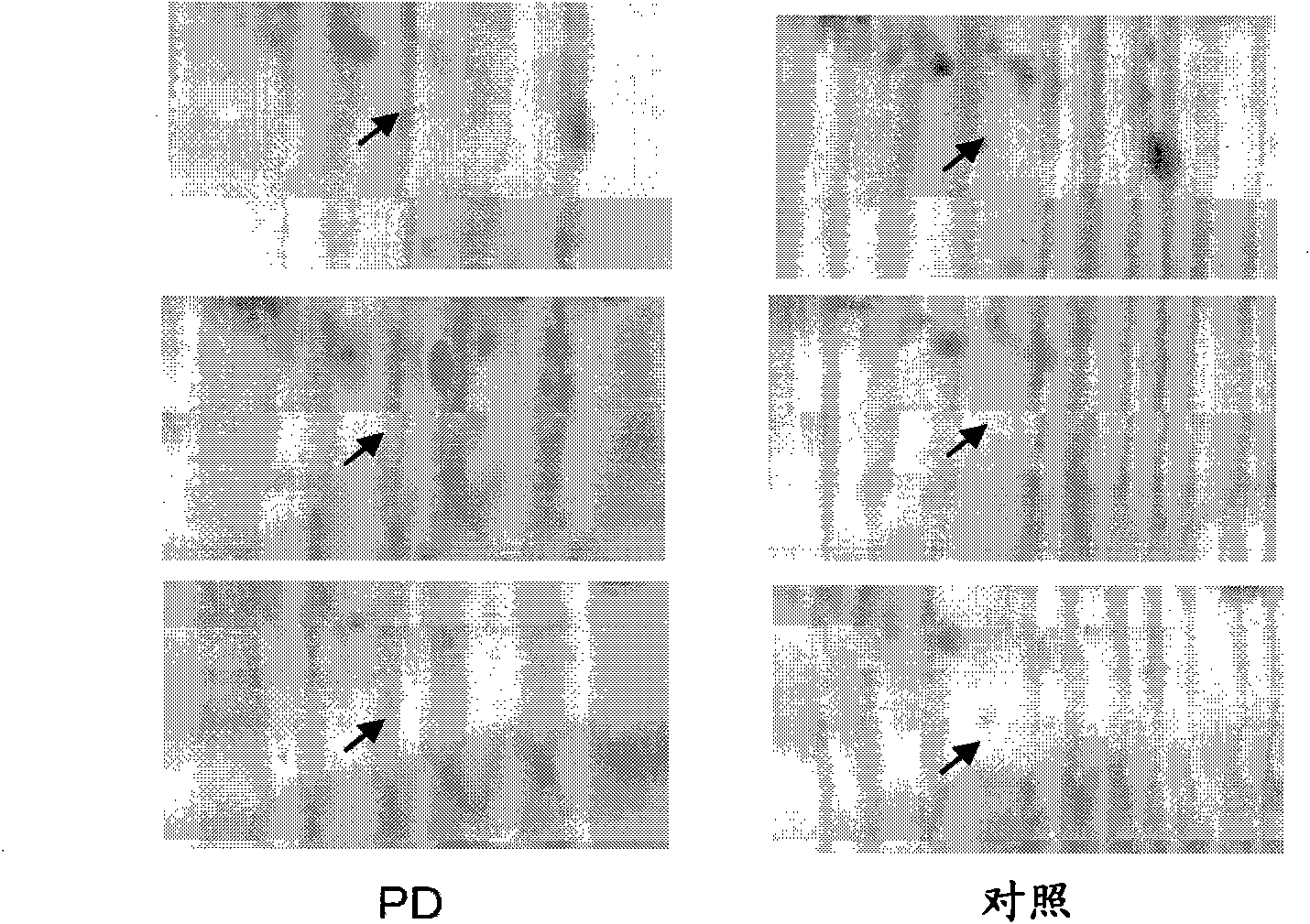
[0109] Example 1 - Separation of proteins and identification of protein variants / isoforms using two-dimensional gel electrophoresis
[0110] Serum protein samples were analyzed by two-dimensional gel electrophoresis. Separation in the first direction was performed by adding 1 mg of protein sample to 24 cm IPG gel strips using a non-linear pH gradient of 3-7. Protein samples were separated using an IPGphor device (GE Healthcare, Piscataway, NJ) at 20°C by pressurizing to 130,000Vh under the following conditions: 40V for 10 hours; 100V for 20 hours; 200V for 2 hours; 500V for 2 hours; 1,000V 2 hours; 8,000V gradient for 2 hours; 8,000V for 12.5 hours. Then the IPG strips were incubated in equilibration buffer (50mM Tris-HCl, 6M urea, 30% (volume / volume) glycerol, 2% (weight / volume) SDS, trace bromophenol blue, 1% (weight / volume) di Thiothreitol) was incubated for 15 minutes. The IPG strips were incubated for an additional 15 minutes in equilibration buffer containing 2.5% (...
Embodiment 2-W
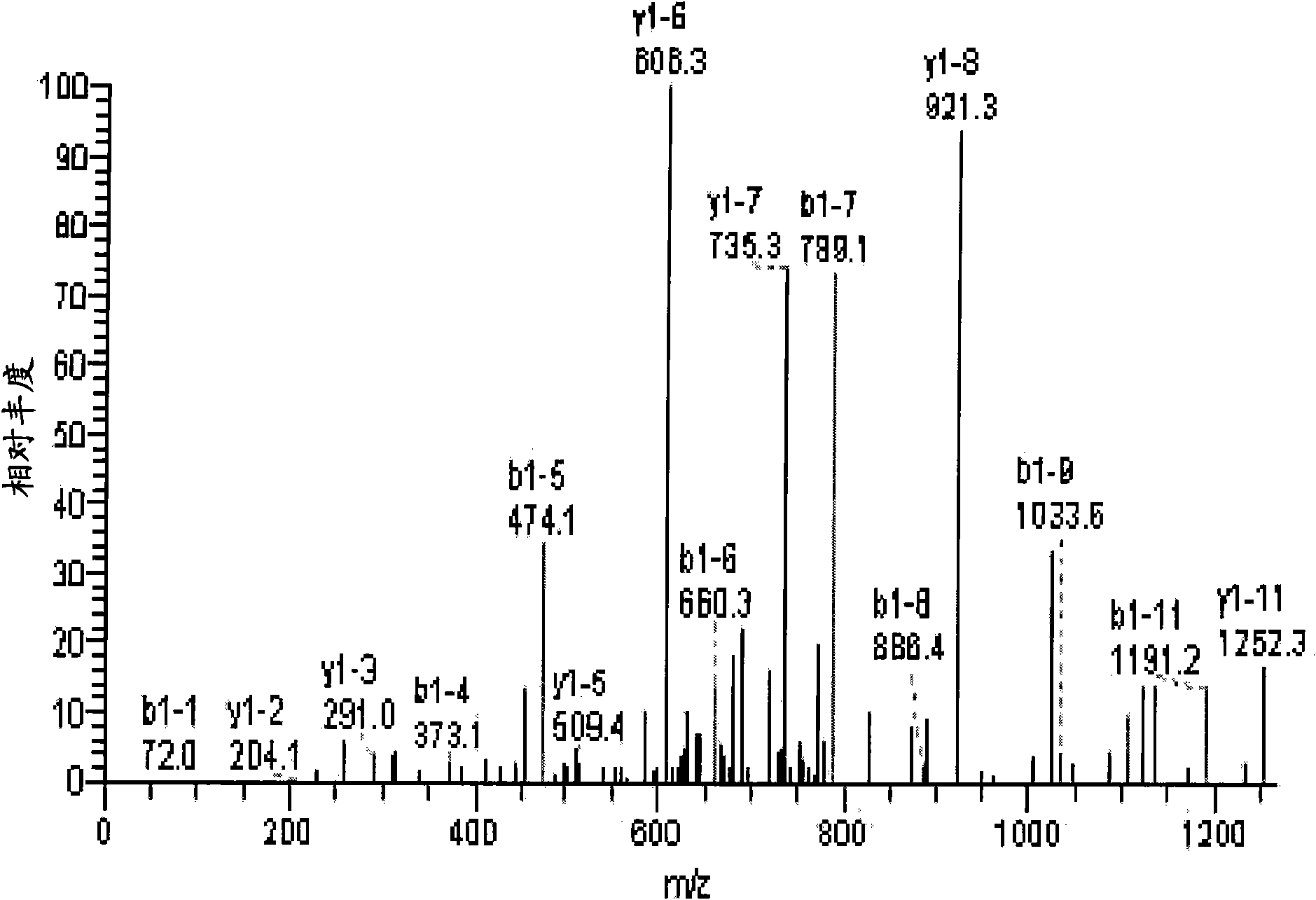
[0114] Example 2 - Western blot validation
[0115] To verify the results obtained in Example 1, sera from PD patients and controls were further subjected to Western blot analysis. Serum protein samples (50 micrograms) were separated by 12.5% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked and incubated overnight at 4°C with TTR polyclonal antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA). Anti-rabbit IgG-HRP secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added at a ratio of 1:10,000. The relative intensities of the bands were calculated with QuantityOne software (Bio-Rad, Hercules, CA, version 4.6.3). Such as Figure 3A As indicated, the presence of TTR was detected in serum proteins (50 mg) of PD patients (lanes 1-3) or controls (lanes 4-6). As a positive control, 50 ng of purified human TTR was added to a separate lane (+). Molecular weight markers are indicated with corresponding KDa masses. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com