Preparing method of 5-bromine pivaloyl bromide normal
A bromide-n-valeryl bromide, a preparation method technology, applied in the field of preparation of organic compounds, can solve the problem of not giving the compound synthesis process, large dosage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
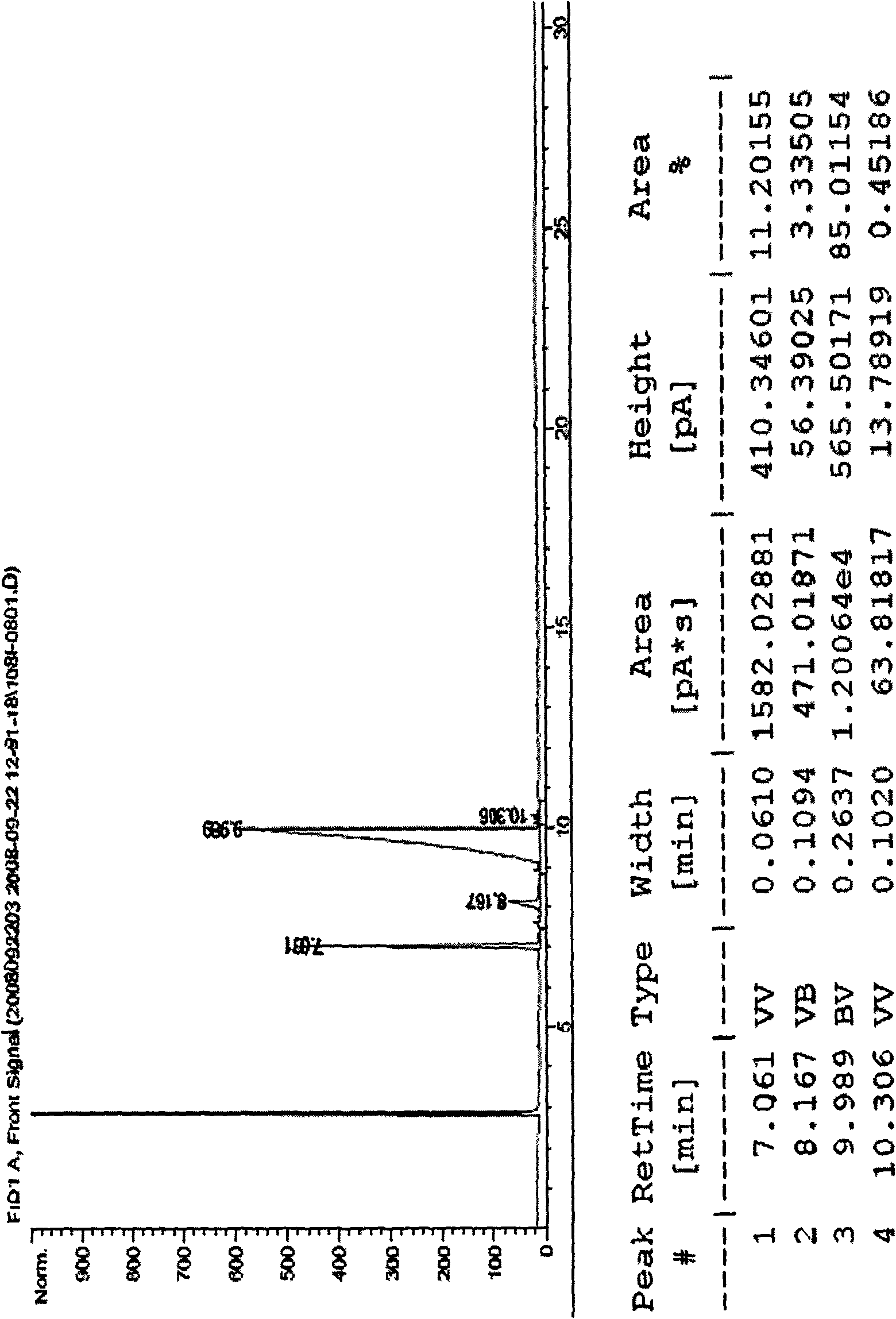
Image
Examples
Embodiment 1
[0018] The preparation method of 5-bromo-n-pentanoyl bromide is specifically divided into three steps, and the reaction formula is as follows:
[0019]
[0020] Synthesis of the first step intermediate I: Add 8.6g (85%, 0.0425mol) of m-chloroperoxybenzoic acid and 19mL of dry chloroform in a 100mL four-necked flask equipped with magnetic stirring, a thermometer and a condenser tube, and add dropwise at room temperature A solution of 2.1 g (0.025 mol) of cyclopentanone in dry chloroform (13 mL). After the dropwise addition, heat to reflux and monitor until the content of cyclopentanone is less than 0.5% to stop the reaction, then perform post-treatment, cool the reaction mixture with an ice-water bath, filter to remove the m-chlorobenzoic acid precipitate, and wash the filtrate with about 10% sodium sulfite aqueous solution until KI- Until the starch test paper does not change color, the solvent is removed by rotary evaporation, and the residue is washed with 10% potassium c...
Embodiment 2-9
[0029] Prepare 5-bromo-n-pentanoyl bromide according to the method described in Example 1, and the process conditions of concrete changes are as follows:
[0030] Synthesis of the first step intermediate I: prepared according to Example 1, or outsourced;
[0031] The synthesis of the second step intermediate II: experiment according to the conditions in the following table:
[0032]
[0033] The synthesis of the third step final product 5-bromo-n-pentanoyl bromide: experiment according to the conditions in the following table,
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com