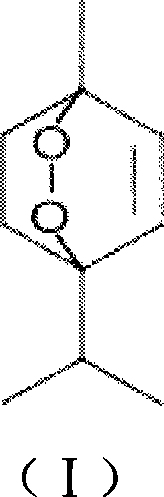Application of ascaridol in preparing medicine for preventing helicobacter pylori and treating disease caused by helicobacter pylori
An anti-Helicobacter pylori, Helicobacter pylori technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 expelling ascarid
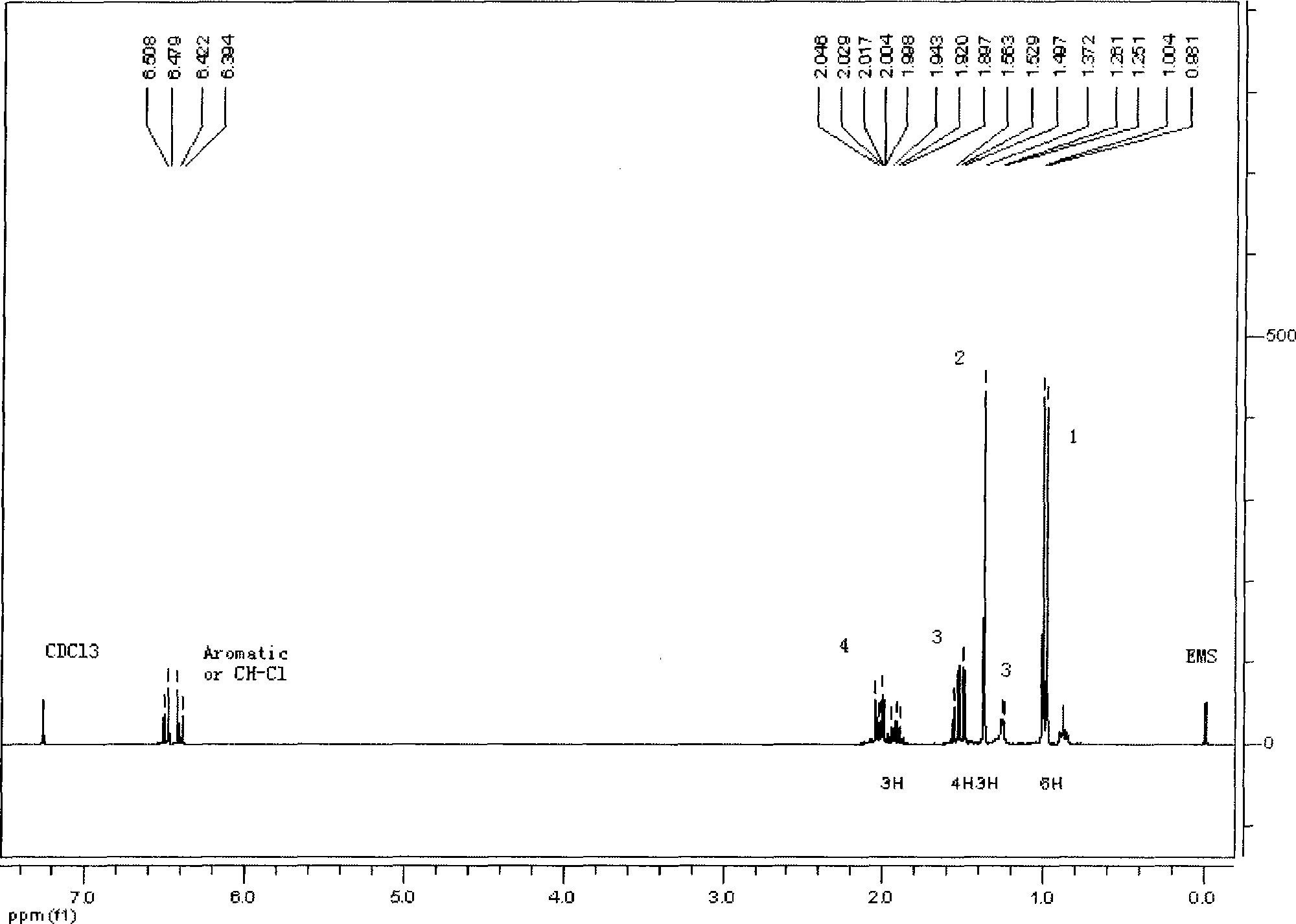
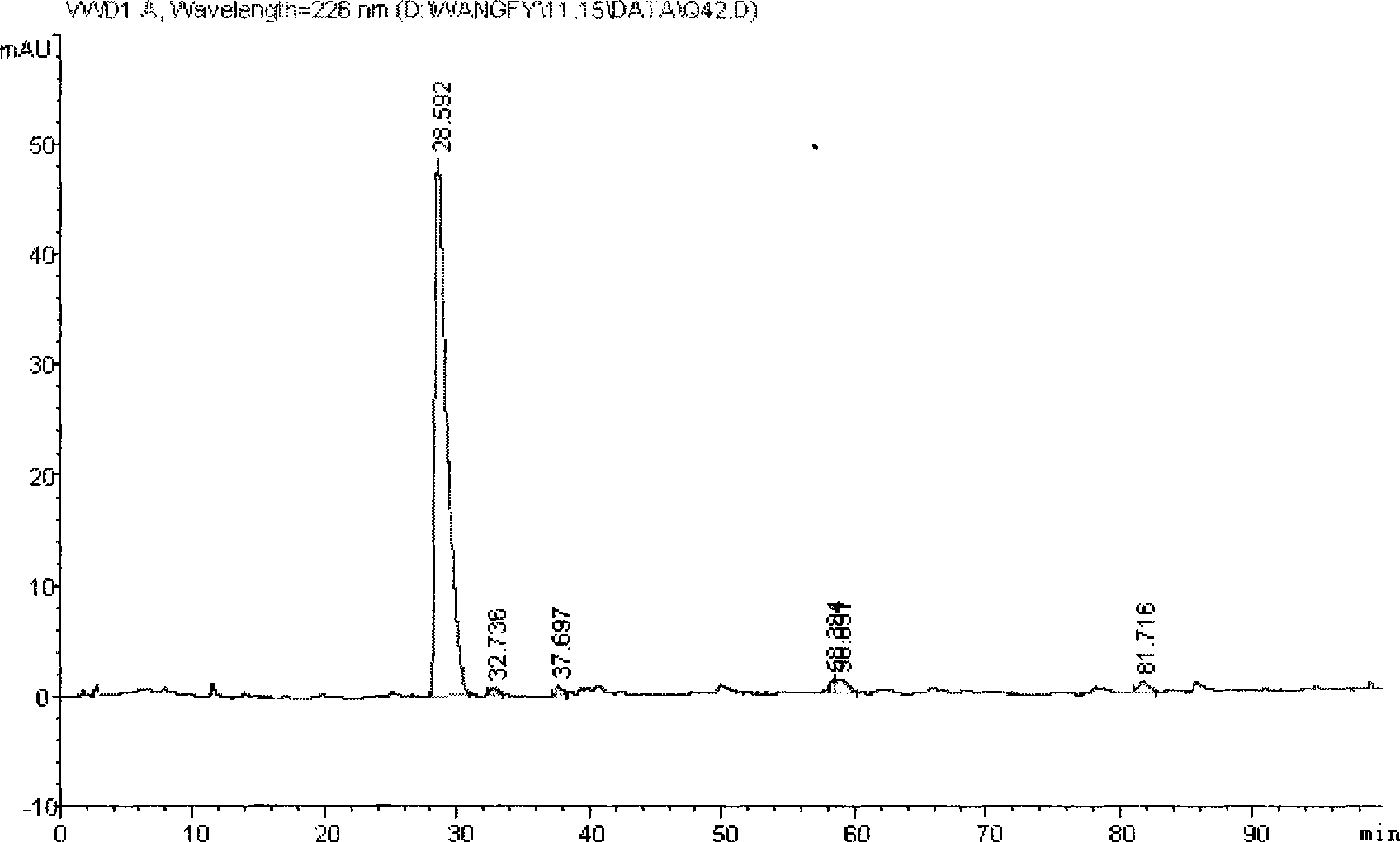
[0046] The volatile oil of Nepeta chinensis is collected and separated by normal-phase silica gel (≤100um) column chromatography, and the amount of silica gel used is 5 to 50 times the weight of the volatile oil. Fraction I was obtained by eluting about 3 to 5 times the retention volume with n-hexane as the starting solvent. Then, it was eluted with n-hexane / ethyl acetate gradient and collected step by step. The sequence is n-hexane / ethyl acetate (100:1) elution about 3 to 5 times the retention volume to obtain Fraction II; n-hexane / ethyl acetate (80:1) elution about 3 to 5 times the retention volume to obtain Fraction III. The content of ascaridin in fraction III is more than 90%. figure 1 shows the extraction of ascaridin in CDCl 3 middle 1 H NMR, the structure of ascaridin extracted from spectrum analysis is correct. figure 2 The HPLC profile of the ascaridin extracted sample is shown. figure 2 Shown is the...
Embodiment 2
[0047] The preparation of embodiment 2 ascarid
[0048] The volatile oil of Nepeta chinensis is collected and separated by normal-phase silica gel (≤100um) column chromatography, and the amount of silica gel used is 5 to 50 times the weight of the volatile oil. Fraction I was obtained by eluting about 5 times the retention volume with petroleum ether (boiling range 30-60° C.) as the starting solvent. Then, it was eluted with petroleum ether / ethyl acetate gradient and collected step by step. The sequence is about 5 times the retention volume of petroleum ether / ethyl acetate (100:1) to obtain fraction II; petroleum ether / ethyl acetate (80:1) elution of about 5 times the retention volume to obtain fraction III. The content of ascaridin in fraction III is more than 90%.
Embodiment 3
[0049] The preparation of embodiment 3 expelling ascarid
[0050] First fully soak the normal-phase silica gel (≤100um) with 2% to 2.5% silver nitrate solution, and then dry it in the dark and shade for later use. The volatile oil of Nepeta chinensis is taken from the soil, and the above-mentioned silver nitrate silica gel is used for column chromatography separation, and the amount of silica gel used is 5 to 50 times the weight of the volatile oil. After elution with n-hexane as the starting solvent for about 3 to 5 times the retention volume, and then gradient elution with n-hexane / ethyl acetate, the samples were collected step by step. The sequence is about 3 to 5 times the retention volume of n-hexane / ethyl acetate (100:1) to obtain fraction I; n-hexane / ethyl acetate (80:1) about 3 to 5 times the retention volume to obtain Fraction II. The content of ascaridin in fraction II is more than 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com