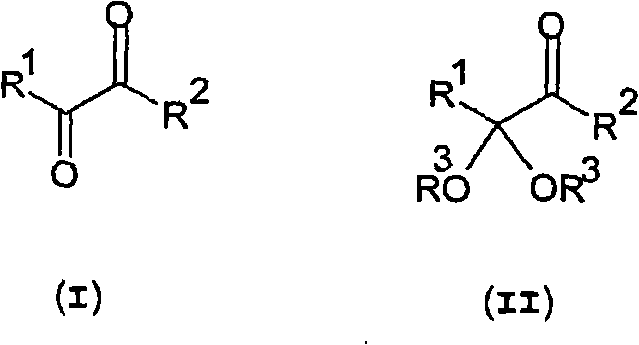
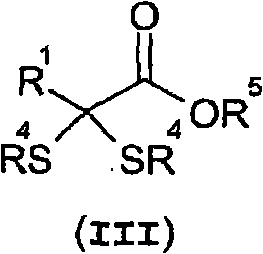
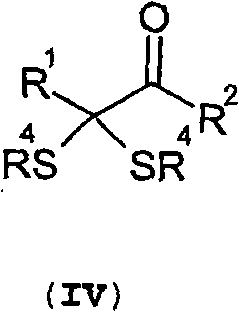
Method for the production of ketoacids and their derivatives
A technology of derivatives and ketoacids, applied in the field of preparation of α-ketoacids, which can solve problems such as difficult separation and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Preparation of 1,1,3-tris(methylthio)propane (2) from 3-(methylthio)propanal (1)
[0080]
[0081] Me = methyl
[0082] 3-(Methylthio)propanal (1) (1.44 mol, 150 g) was saturated with HCl (g) at 0°C, then added directly to MeSH (6.25 mol, 300 g) dropwise over 30 minutes at 0°C )middle. The reaction mixture was heated to 20 °C and stirred at 20 °C for 24 h. After removal of excess MeSH under reduced pressure, GC analysis showed the following product distribution: 91% thioacetal (2) and 8% 3-(methylthio)propanal (1). For further purification, the mixture was dissolved in ether and treated with 30% sodium metabisulfite (Na 2 S 2 o 5 ) aqueous solution washing. After phase separation, MgSO 4 The organic phase is dried. After removal of the solvent, the thioacetal (2) was obtained as a pale yellow oil (168 g, yield = 64%, GC purity = 98%).
[0083] 1,1,3-tris(methylthio)propane (2) 1 H NMR (500MHz, CDCl 3 ): δ=2.01-2.05 (m, 2H, CH 2 ), 2.11(s, 9H, 3×SCH 3 ), ...
example 2
[0086] Preparation of 1,1,3-tris(methylthio)propane (2) from acrolein (3)
[0087]
[0088] Acrolein (3) (89 mmol, 5.0 g) was added dropwise to MeSH (358 mmol, 17.2 g) which had been saturated with HCl gas at 0 °C over 15 minutes under stirring at -78 °C. This mixture was heated slowly to 20°C and then stirred at 20°C for a further 24h. After removal of excess MeSH under reduced pressure, the mixture was dissolved in ether and washed with 30% aqueous sodium metabisulfite. After phase separation, MgSO 4 The organic phase is dried. After distillation (88° C. at 1.2 mbar), pure thioacetal (2) was obtained as a pale yellow oil (11.4 g, yield=70%, GC purity=98%). NMR characterization gave the same data as in Example 1.
Embodiment 3
[0090] Through 1,1,3-tris(methylthio)propane (2) and dry ice (CO 2 ) to prepare 2,2,4-tri(methylthio)butanoic acid (4).
[0091]
[0092] In a three-neck flask under a protective gas atmosphere, 1,1,3-tris(methylthio)propane (2) (3.65 g, 20 mmol) was dissolved in 50 ml of anhydrous THF. Subsequently, a solution of butyllithium in n-hexane (14ml, 1.6M) was slowly added dropwise under stirring at -20°C. During this time, the solution turned bright yellow. After continuing to stir at this temperature for 2 h, anhydrous dry ice (CO 2 , 10g). The reaction solution was slowly thawed to 20° C., and 10% aqueous KOH (80 ml) was added. After phase separation, the organic phase was washed with 10% aqueous KOH (2 x 50 ml). The combined KOH phases were washed with diethyl ether (3 x 30 ml) and carefully adjusted to pH=1 with concentrated HCl while cooling. The product was extracted with ether (3 x 50ml). followed by Na 2 SO 4 The combined ether phases were dried, filtered and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com