Surface bioactive calcium-phosphate layer of carbon fiber reinforced polyetheretherketone composite material and preparation thereof
A technology of bioactive calcium and polyetheretherketone, applied in coatings, medical science, prostheses, etc., can solve problems such as high application value, and achieve the effects of improving biological level, good therapeutic effect, and good surface biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
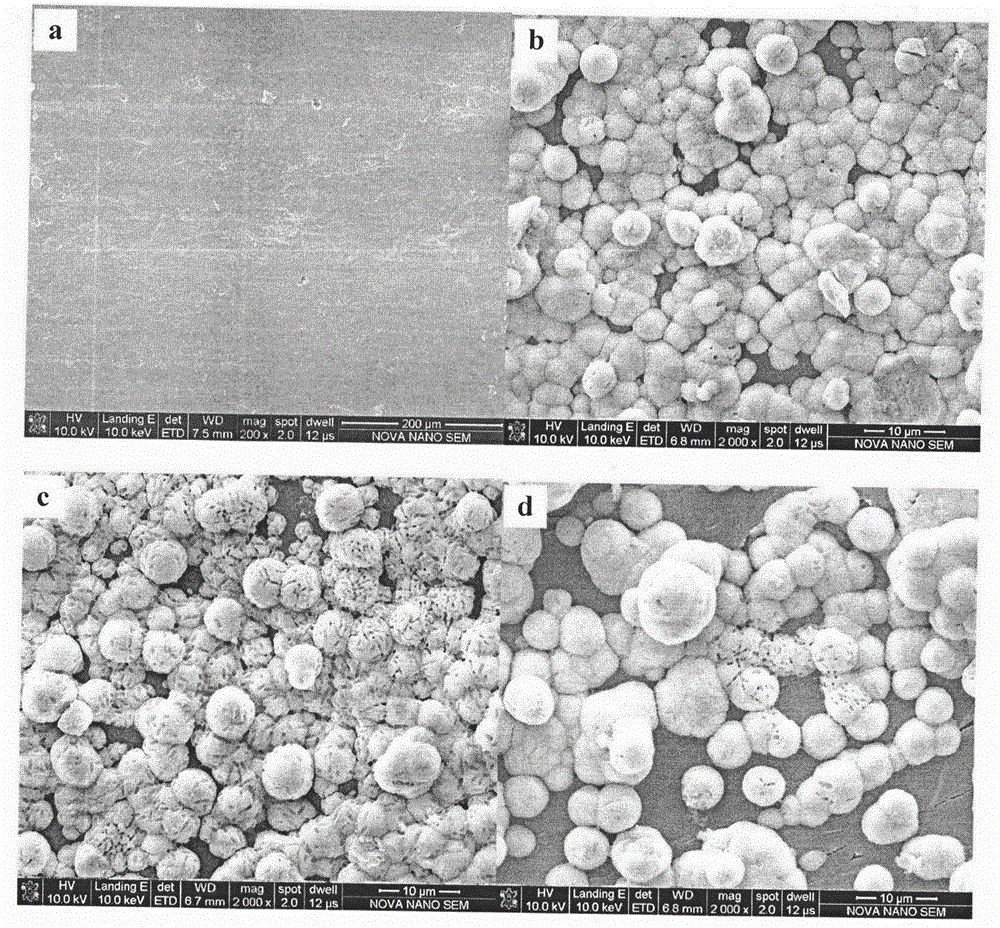
[0025] The preparation method of the three-dimensional braided carbon fiber reinforced polyether ether ketone composite material is as follows: first perform three-dimensional and four-directional weaving of carbon fiber and polyether ether ketone fiber, and then heat the fiber fabric to 140-180°C, continue to vacuum, and heat for 1.5 hours, then Raise the temperature to 380°C at a rate of 20°C / min and keep the temperature for 30 minutes. Press 0.5MPa again, remove the vacuum, continue to maintain the temperature for 30 minutes, and finally lower the temperature slowly, and release the mold when the temperature is lower than the glass transition temperature of polyetheretherketone by 30°C. The selected carbon fiber is PAN-based carbon fiber with a tensile strength of 2800MPa, a tensile modulus of 200GPa, and a density of 1.75g / cm 3 , With a diameter of 6-8μm, produced by Jilin Carbon Factory.
[0026] The three-dimensional braided carbon fiber reinforced polyether ether ketone co...
example 2
[0028] The three-dimensional woven carbon fiber reinforced polyether ether ketone composite material with a fiber volume content of 54% (the preparation method is the same as in Example 1) was ultrasonically cleaned with acetone, absolute ethanol, and deionized water at room temperature for 10 minutes, and then dried. Place it in an atmospheric reactor, adjust the frequency to about 8kHz, output voltage about 8kV, current about 40mA, and discharge time of 120s. Then at 37.5℃, the composite material was placed in 1.5 times SBF simulated body fluid for biomimetic mineralization. The standard simulated body fluid ion solubility is [Na + ]: 142.0mM, [K + ]: 5.0mM, [Mg 2+ ]: 2mM, [Ca 2+ ]: 2.5mM, [Cl - ]: 147.8mM, [HCO 3 - ]: 4.2mM, [HPO 4 2- ]: 1.0mM, [SO 4 2- ]: 0.4mM, and trihydroxyaminomethyl methane (CH 2 OH) 3 CNH 2 And HCl solution is a slow release agent to adjust the pH of the solution to 7.4. Take it out after 28 days of biomimetic mineralization. The simulated body fluid...
example 3
[0030] The three-dimensional woven carbon fiber reinforced polyether ether ketone composite material with a fiber volume content of 36% (the preparation method is the same as in Example 1) was ultrasonically cleaned with acetone, absolute ethanol, and deionized water at room temperature for 10 minutes, and then dried. It is placed in the MEVVA source ion implanter, with a 99% purity Ti rod as the working electrode, which is implanted facing the Ti ion source. The injected beam current density is 0.4~0.5mA·cm -2 , The arc voltage is 70V, the trigger pressure is 80V, and the air pressure in the target chamber is about 8×10 -4 Pa, the injection dose is 5.0×10 15 . Then at 37.5℃, the composite material was placed in 1.5 times SBF simulated body fluid for biomimetic mineralization for 7 days. The standard simulated body fluid ion solubility is [Na + ]: 142.0mM, [K + ]: 5.0mM, [Mg 2+ ]: 2mM, [Ca 2+ ]: 2.5mM, [Cl - ]: 147.8mM, [HCO 3 - ]: 4.2mM, [HPO 4 2- ]: 1.0mM, [SO 4 2- ]: 0.4mM, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| impact strength | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com