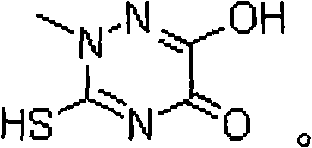
Method for synthesizing triazine ring
A synthesis method and technology of triazine ring, applied in the field of synthesis of triazine ring, can solve the problems of high cost of raw materials, complicated operation, low yield and the like, and achieve the effects of low cost of raw materials, high yield, and reduction of three-waste pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] ①Put 40g of methylhydrazine aqueous solution and 29g of ammonium thiocyanate into a 500ml reaction flask, heat to reflux, and control the reaction temperature at 85-90°C. After removing the water under negative pressure, add 180g of methanol to obtain aminomethylhydrazine methanol solution .
[0029] ②Add 50g dimethyl oxalate and 118g sodium methoxide to the reacted aminomethylhydrazine methanol solution, heat and reflux for 6 hours, control the reaction temperature at 65-75°C, and adjust the pH value to 6-7 with hydrochloric acid to remove excess sodium methoxide, filtered, and the filtrate (triazine ring sodium salt solution) was acid-absorbed with hydrochloric acid to adjust the pH value to 1-2, and filtered to obtain a crude triazine ring.
[0030] ③The crude triazine ring was added to hot water, stirred, cooled and crystallized, filtered, and dried to obtain 41 g of triazine ring with a yield of 70%.
Embodiment 2
[0032] ①Put 40g of methylhydrazine aqueous solution and 29g of ammonium thiocyanate into a 500ml reaction flask, heat to reflux, and control the reaction temperature at 95-100°C. After removing the water under negative pressure, add 200g of methanol to obtain aminomethylhydrazine methanol solution.
[0033] ②Add 52g dimethyl oxalate and 122g sodium methoxide to the reacted aminomethylhydrazine methanol solution, heat and reflux for 6 hours, control the reaction temperature at 65-75°C, adjust the pH value to 6-7 with hydrochloric acid after the reaction, and filter , the filtrate is then adjusted to a pH of 1 to 2 with hydrochloric acid, filtered, and the filter cake is the crude product of the triazine ring.
[0034] ③ Add the crude triazine ring to an appropriate amount of hot water, stir, cool and crystallize, filter, and dry to obtain 44 g of the triazine ring with a yield of 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com