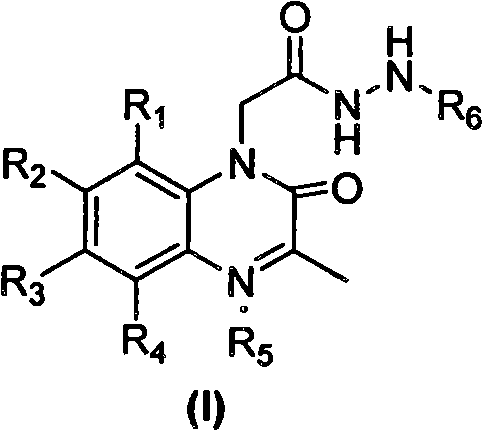
Quinoxalinone derivative with matrix metalloproteinase inhibitory activity and preparation method and application thereof
A technology of protease inhibition and quinoxalinone, applied in the direction of organic active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve inappropriate problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The preparation method of 2-(3-methyl-2-oxo-quinoxaline-1-(2H)-yl)acetohydrazide (lyg-31 in table 1)
[0076]Weigh o-phenylenediamine (10.81g, 0.10mol), add it to 250ml of absolute ethanol, stir at room temperature for 15min until dissolved, add ethyl pyruvate (12.77g, 0.11mol), a yellow solid is formed, continue to stir for 4h, react complete. Suction filtration gave a light yellow solid, which was recrystallized from ethanol to obtain 3-methylquinoxaline-2(1H)-one with a yield of 92.7%, m.p.241-243°C; weigh 3-methylquinoxaline-2 (1H)-ketone (8.01g, 0.05mol), anhydrous potassium carbonate (8.29g, 0.06mol) and ethyl chloroacetate (7.353g, 0.06mol), add 200ml of acetone to form a suspension, add tetrabutyl Ammonium bromide (0.5g, cat.), heated to reflux under oil bath conditions, TLC detected the completion of the reaction, evaporated the solvent under reduced pressure, added 100ml of water and 200ml of ethyl acetate for extraction three times, spin-dried ethyl acetate ...
Embodiment 2
[0078] The preparation method of N-(2-3-methyl-2-oxo-quinoxalin-1-(2H)-yl) acetyl) phenylacetylhydrazide (lyg-32 in table 1)
[0079] Weigh o-phenylenediamine (10.81g, 0.10mol), add it to 250ml of absolute ethanol, stir at room temperature for 15min until dissolved, add ethyl pyruvate (12.77g, 0.11mol), a yellow solid is formed, continue to stir for 4h, react Complete. Suction filtration to obtain a light yellow solid, recrystallized from ethanol to obtain 3-methylquinoxaline-2(1H)-one, the yield is 92.7%, m.p.241-243°C; weigh 3-methylquinoxaline -2(1H)-ketone (8.01g, 0.05mol), anhydrous potassium carbonate (8.29g, 0.06mol) and ethyl chloroacetate (7.353g, 0.06mol), add 200ml of acetone to form a suspension, add four Butylammonium bromide (0.5g, cat.) was heated to reflux under oil bath conditions, TLC detected that the reaction was complete, the solvent was evaporated under reduced pressure, 100ml of water and 200ml of ethyl acetate were added for extraction three times, and ...
Embodiment 3
[0081] 2,6-dichloro-N'-(2-(3-methyl-2-oxo-quinoxalin-1-(2H)-yl)acetyl)phenylacetylhydrazide (lyg-34 in Table 1) Preparation
[0082] See Example 1 for the preparation of 2-(3-methyl-2-oxo-quinoxalin-1-(2H)-yl)acetylhydrazide. Weigh 2-(3-methyl-2-oxo-quinoxalin-1-(2H)-yl)acetohydrazide (0.23g, 1mmol), anhydrous sodium carbonate (0.12g, 1.1mmol), add 20ml anhydrous Then add 2,6-dichlorobenzoyl chloride (0.209g, 1mmol) into water tetrahydrofuran, stir at room temperature for 12h, and the reaction is complete. The solvent was evaporated under reduced pressure, washed with a small amount of water, and filtered; the filter cake was recrystallized with ethanol to obtain white powder 2,6-dichloro-N'-(2-(3-methyl-2-oxo-quinoxaline- 1-(2H)-yl)acetyl)phenylacetylhydrazide, yield 57.1%, m.p.294-296°C, ESI-MS 405.8(M+H), 1 H-NMR: (DMSO-d 6 , ppm) δ: 2.43 (s, 3H, CH 3 ), 5.048 (s, 2H, NCH 2 CO), 7.355-7.38 (m, 2H, ArH), 7.46 (d, J=8.4Hz, 1H, ArH), 7.517-7.582 (m, 2H, ArH), 7.72 (d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com