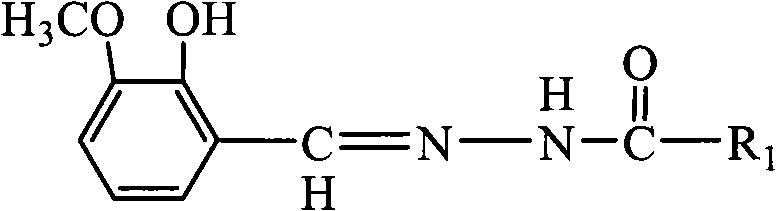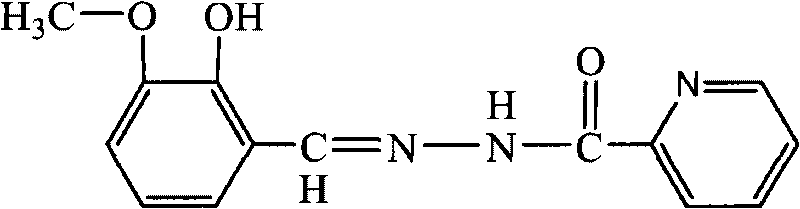Preparation method of substituted pyridine carbonylhydrazone metal corrosion inhibitor
A metal corrosion inhibitor and a picolinyl hydrazone technology, which is applied in the field of preparation of a substituted picolinyl hydrazone metal corrosion inhibitor, can solve the problems of high hazard, large amount of corrosion inhibitor, unreasonable molecular design, etc. Good corrosion inhibition performance and good biodegradability
Inactive Publication Date: 2010-05-19
GUILIN UNIVERSITY OF TECHNOLOGY
View PDF0 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
For example, the amount of corrosion inhibitor is large, the cost is high, the molecular design is unreasonable, the synthesis route and process are complex, the production of harmful industrial by-products, the yield is low, and the quality is poor, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
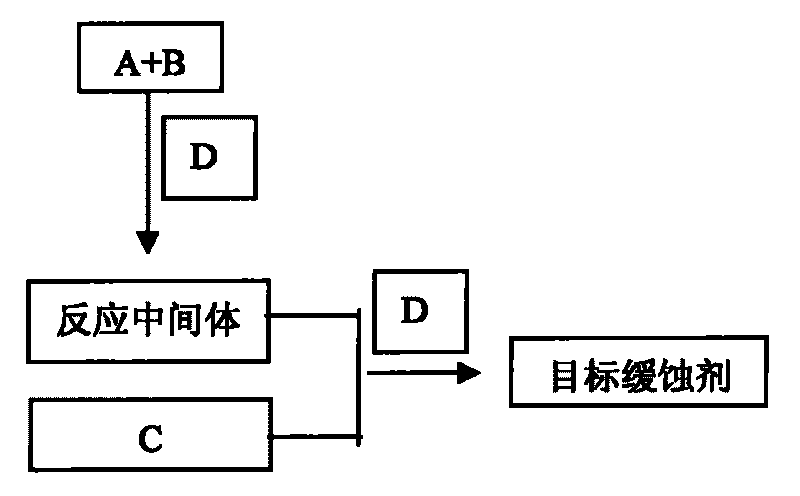
The invention discloses a preparation method of substituted pyridine carbonylhydrazone metal corrosion inhibitor. The method comprises the following steps: using methylisonicotinate and hydrazine hydrate in a molar ratio of 1:1-1.2:1 and absolute ethanol or methanol used as solvent of which volume is 3-6 times of hydrazine hydrate to react for 6-8h in a three-neck flask under the condition of refluxing at 70-80 DEG C and magnetic stirring, obtaining a product to perform reduced pressure distillation, recrystallizing with absolute ethanol, drying to obtain pyridine formylhydrazine, then using the obtained pyridine formylhydrazine and o-vanillin in a molar ratio of 1:1-1:1.2 and absolute ethanol or methanol used as solvent of which volume is 2-5 times of pyridine formylhydrazine to react for 7-9h under the condition of refluxing at 70-90 DEG C and magnetic stirring, cooling, performing suction filtration, washing with absolute ethanol, and recrystallizing. The preparation method of the invention designs the corrosion inhibitor molecule with good oil solubility which meets the environmental protection requirements; and the corrosion inhibitor molecule is characterized by good biodegradability, low toxicity and corrosion resistance within the pH value range of 4-9, and has good corrosion resistance to A3 carbon steel and copper.
Description
technical field The invention relates to a substituted pyridineformylhydrazone compound for metal material protection, belongs to the field of molecular design of green corrosion inhibitors, and particularly relates to a preparation method of a substituted pyridineformylhydrazone metal corrosion inhibitor. Background technique Compared with the most commonly used inorganic corrosion inhibitors (nitrite, phosphate, polyphosphate, carbonate, silicate, borate, chromate), there are more types of synthetic organic corrosion inhibitors . However, there are still many deficiencies in the molecular structure design, synthesis route and process, and application performance of organic corrosion inhibitors, and its theoretical progress still lags far behind practice. The selection and design of the molecular structure of green corrosion inhibitors must not only consider the corrosion inhibition effect, but also take into account the biological activity and versatility. In order to ad...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C23F11/14
Inventor 刘峥王国瑞刘宝玉陈世亮
Owner GUILIN UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com