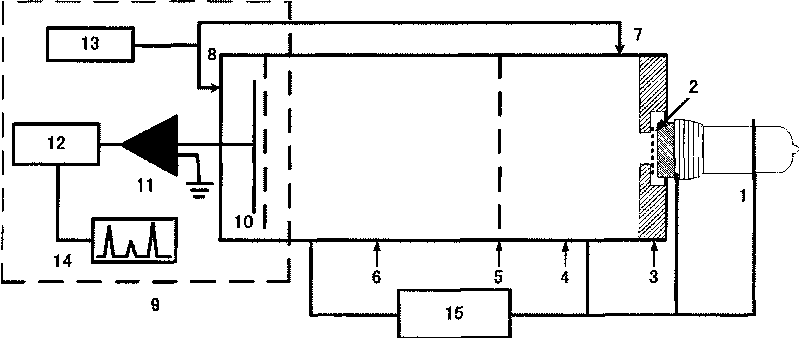
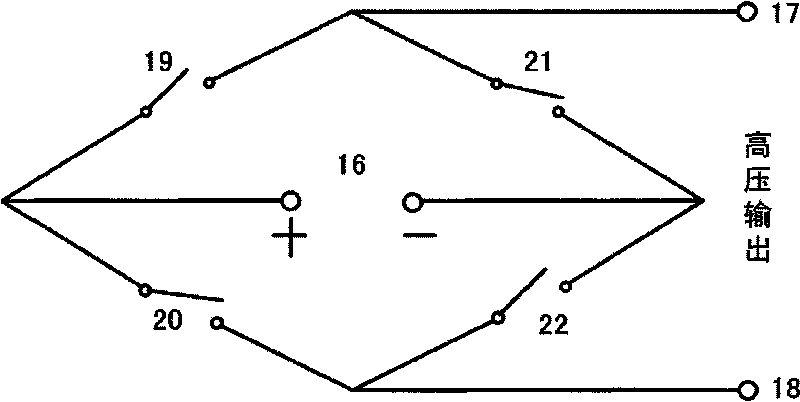
Method for identifying and detecting halogenated hydrocarbons
A technology for compounds and halogenated hydrocarbons, applied in the field of analysis of halogenated hydrocarbon compounds, can solve the problems of difficult separation and detection of compounds, and achieve the effect of real-time online analysis, small instrument size and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
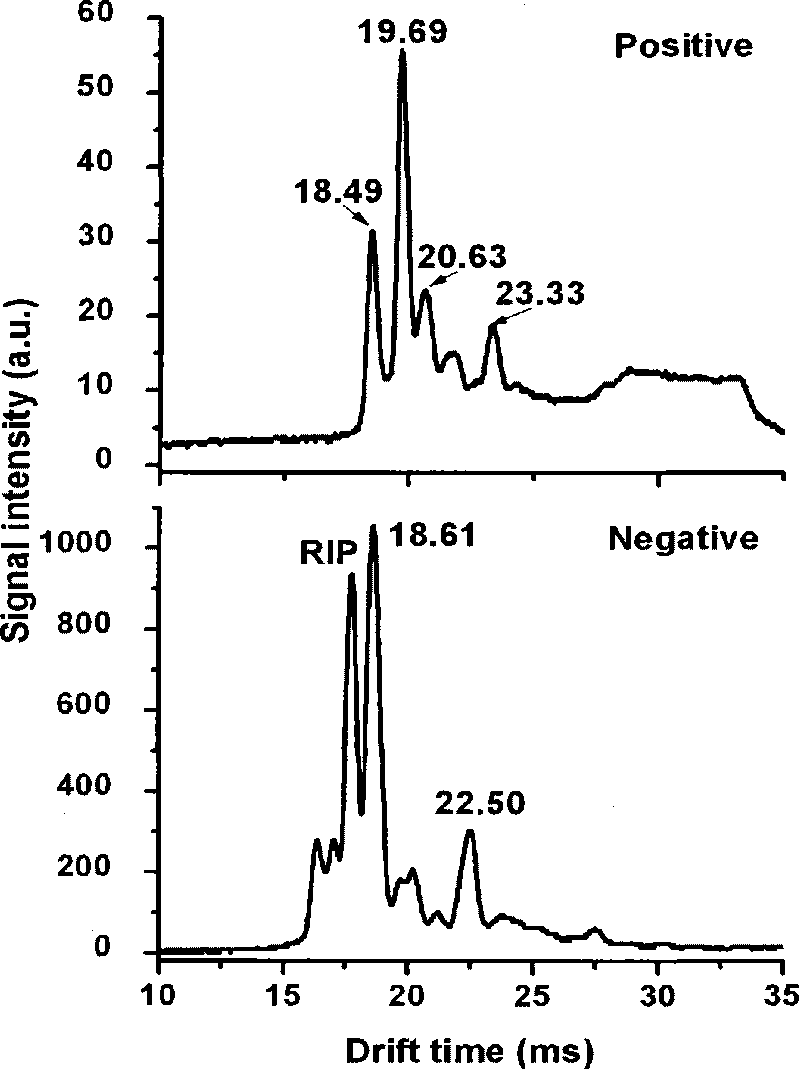
[0037] figure 2 The detection spectra of TCE in positive and negative ion modes are given. In the figure, RIP represents the reagent ion peak, here CO 3 - (H 2 O) n . It can be seen from the figure that TCE can be well detected in both positive and negative ion modes. In the positive ion mode, it has four characteristic peaks, and its migration time is 18.49ms, 19.69ms, 20.63ms and 23.33ms respectively. In the negative ion mode, it has Cl - In addition to the characteristic peak (18.61ms), it also has its own characteristic peak with a migration time of 22.50ms, so it can be well separated from the reagent ion peak.
Embodiment 2
[0039] image 3 is given by CCl 4 Detection spectra in negative ion mode. It can be seen from the figure that CCl 4 With Cl in negative ion mode - The characteristic peak (18.69ms). Due to CCl 4 The ionization energy of the ionization energy is greater than that of ultraviolet light, and it cannot be ionized in the positive ion mode, and its spectrum cannot be obtained. In this way, it can be distinguished from the TCE, and at the same time, its interference to the detection of the TCE can be eliminated.
Embodiment 3
[0041] The experimental conditions are the same as those mentioned above Figure 2-3 The experimental conditions used in the experimental spectrum are the same; in the experiment, it is measured that the detection limit of the ultraviolet photoionization source ion mobility spectrum to carbon tetrachloride is 4.3 × 10 -9 g, the detection limit of tetrachlorethylene is 5.0×10 -11 g, thus it can be seen that the ionization energy of carbon tetrachloride and tetrachlorethylene is 11.47eV and 9.326eV, the former is higher than the ionization energy (10.6eV) of ultraviolet light, and the latter is lower than the ionization energy of ultraviolet light, so four Chlorinated carbon cannot undergo photoionization, but tetrachlorethylene can undergo photoionization to generate a large number of electrons, thereby greatly improving the detection sensitivity of ion mobility spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com