Method for acquiring solid tumor cell with successful in-vitro transfection
A technology for solid tumors and cells, which is applied to cells modified by introducing foreign genetic material, biochemical equipment and methods, and the determination/inspection of microorganisms. It can solve the problems of low transfection efficiency and unstable experimental results. Improved therapeutic efficacy, simple operation, and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Establishment of a three-dimensional growth solid tumor cell model
[0032] Coated Poly-HEMA culture plate
[0033] Dissolve 2.4g of Poly-HEMA powder in 20mL of 95% ethanol, shake and mix at 65°C for more than 8h, prepare Poly-HEMA storage solution, and store in a sealed container at 4°C. Dilute the working solution with 95% ethanol at a ratio of 1:2.3. Under sterile conditions, gently add the working solution to the bottom of the well of the culture plate along the well wall to cover the whole bottom of the well, and then irradiate with ultraviolet light in the ultra-clean bench overnight. , that is, the Poly-HEMA culture plate was prepared, covered and stored at 4°C for one week, irradiated with ultraviolet light for 0.5h before use, and washed 3 times with sterile PBS.
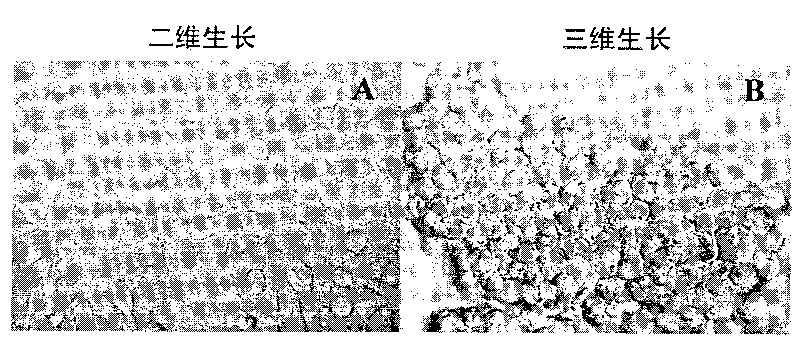
[0034] Establishment of 3D growing solid tumor cell models
[0035] 1) The BEL7402 cell line was cultured with RPMI1640 plus 10% fetal bovine serum at 37°C and 5% CO 2 Cultured under condi...
Embodiment 2
[0043] Example 2 Direct transfection of antisense oligonucleotides into two-dimensional and three-dimensional growing cells
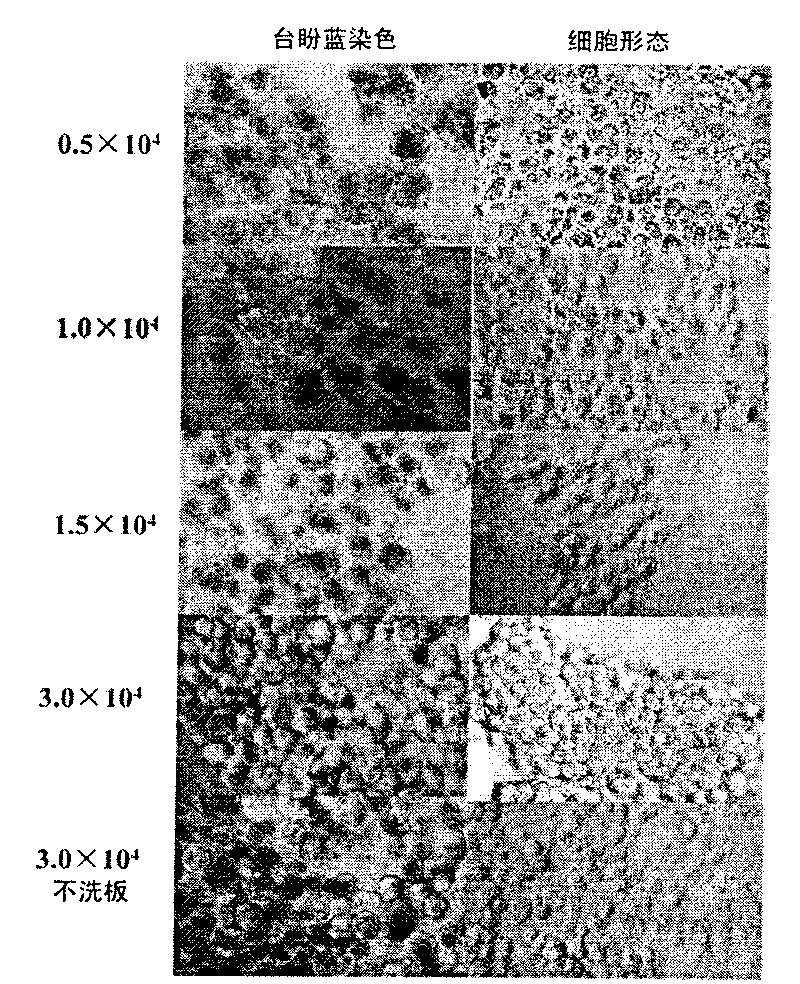
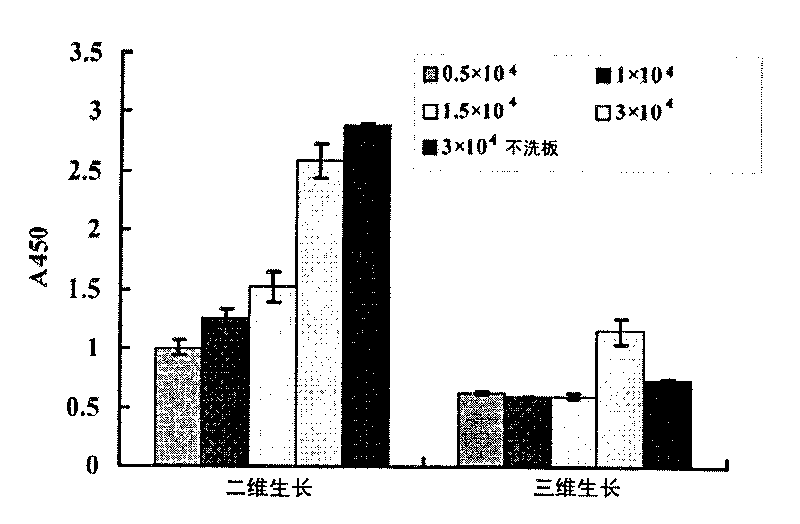
[0044] 1) BEL7402 cells were treated with 3×10 per well 5 The concentration of cells was inoculated on a 24-well plate, wherein the bottom of the hole was covered with Poly-HEMA (i.e. "Poly-HEMA culture plate" in Example 1) was a three-dimensional growth group, and the group without Poly-HEMA was a two-dimensional growth group. Three wells were repeated in each group, and the cells were routinely cultured for 20 hours.
[0045] 2) Add PS-asODNs / ANGPTL4 or PS-rODNs (final concentration: 10 μmol / L) to each well, 37°C, 5% CO 2 After culturing for 24 hours, the cells were collected in 0.5mL TRIZOL.
[0046] 3) Extracting total cellular RNA, and obtaining cDNA through reverse transcription reaction.
[0047] 4) Using the obtained cDNA as a template, PCR amplifies ANGPTL4 and the internal reference gene β-actin.
[0048] 5) 10 μL of the PCR reaction produ...
Embodiment 3
[0050] Example 3 Liposome-mediated transfection of antisense oligonucleotides into two-dimensional and three-dimensional growing cells
[0051] 1) with embodiment 2.
[0052] 2) Mix the antisense oligonucleotide mixture, take 6 μL of PS-asODNs / ANGPTL4 or PS-rODNs antisense oligonucleotide and add 145 μL of Optim MEM and mix well.
[0053] 3) To dilute liposomes, take 29 μL lipofectamine 2000 and add 461 μL Optim MEM, mix well and place at room temperature for 5 minutes.
[0054] 4) Mix 151 μL (2) and 151 μL (3) evenly, let stand at room temperature for 20 minutes, then add 50 μL / well to each well of PS-asODNs / ANGPTL4 group or PS-rODN group, and add 151 μL (3) to each well of Optim MEM to mix Add 50 μL / well to each well of the liposome control group, and set up a blank control group, add the same volume of Optim MEM, mix well, 37 ° C, 5% CO 2 Continue to culture for 24h, collect the cells in 0.5mL TRIZOL.
[0055] 5) All the other steps are the same as in Example 2.
[0056...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com