Desmodium-capillary artemisia cholecystagogue, preparation method and quality control method thereof
A kind of drug, the technology of Yinchen, applied in the field of Jinyin choleretic drugs, can solve the problems such as affecting the therapeutic effect, the dosage is not obvious, and the stomach hurts the spleen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Screening experiment of medicinal flavor compatibility synergy and medicinal flavor ratio
[0028] Using p-xylene-induced ear swelling in mice and the effect on bile secretion in mice, the anti-inflammatory and choleretic effects of Desmodium syringae alone and in combination with capillary wormwood were investigated. The results are shown in Table 1. Dosage is based on the provisions of "Chinese Pharmacopoeia" 2005 Edition, Part One, Desmodium chinensis [Usage and Dosage] 15-60g; Capillaris [Usage and Dosage] 6-15g.
[0029] Table 1: Experiments on the effects of the combination of Desmodium and capillary and the use of p-xylene in different proportions on ear swelling in mice and bile secretion in mice
[0030]
[0031] It can be known from the above table: In terms of the effect on bile secretion (the larger the value, the better) and the anti-inflammatory effect of p-xylene on the ear swelling of mice (the smaller the value, the better), Desmodium and ...
Embodiment 2
[0033] Embodiment 2: extraction process screening experiment
[0034] In this group of examples, 1000g of Desmodium chinensis and 500g of capillary wormwood were put into each experiment, and extracted according to the scheme in the table below. The results are shown in Table 2 and Table 3, and 3 batches of verifications have been carried out according to the extraction scheme screened out, and the extraction process of this invention patent has been obtained: the medicinal materials are put in at a ratio of 2: 1 for Desmodium sativa and Capsicum mellifera, and each addition 10 times the amount of water, decoct three times, 1.5 hours each time, filter the decoction, combine the filtrates, concentrate under reduced pressure to an extract with a relative density of 1.10-1.20.
[0035] Table 2: Factor Level Table
[0036]
[0037] Table 3: Orthogonal experiment design table and results
[0038]
[0039] Analysis of variance will be used in the results of the orthogonal ex...
Embodiment 3
[0043] Embodiment 3: Extraction purification process screening experiment
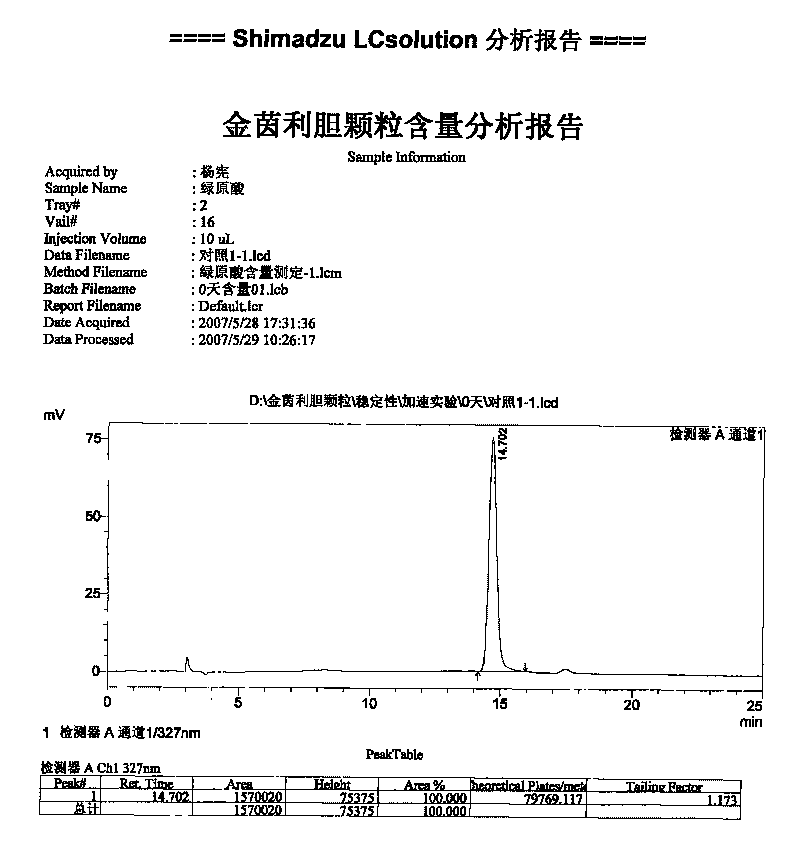
[0044] In this group of examples, the clear paste is obtained by extracting the best extraction process screened out, and then press L 9 (3 4 ) Orthogonal experimental design table for alcohol precipitation, filtration. Ethanol was recovered from the filtrate under reduced pressure, and the content of chlorogenic acid in the obtained extract was measured, and the amount of chlorogenic acid in the extract was calculated as an evaluation index.
[0045] Table 5 Factor level table
[0046]
[0047] Form 6L 9 (3 4 ) Orthogonal test design table and results
[0048]
[0049]
[0050] The results of the orthogonal experiment were analyzed by variance, and the results are shown in Table 7
[0051] Table 7 variance analysis table
[0052]
[0053] As can be seen from the results in the table above, its main influencing factors are A (the ethanol concentration of the medicinal solution), foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com