(S)-amino acid Schiff base coordination compound, preparation method and application thereof
A technology of alanine Schiff base and dimethyl butyric acid Schiff base is applied in the field of preparation of amino acid Schiff base complexes, can solve problems such as application limitation of chiral prosthetic group II, and achieves low cost and simple method , the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
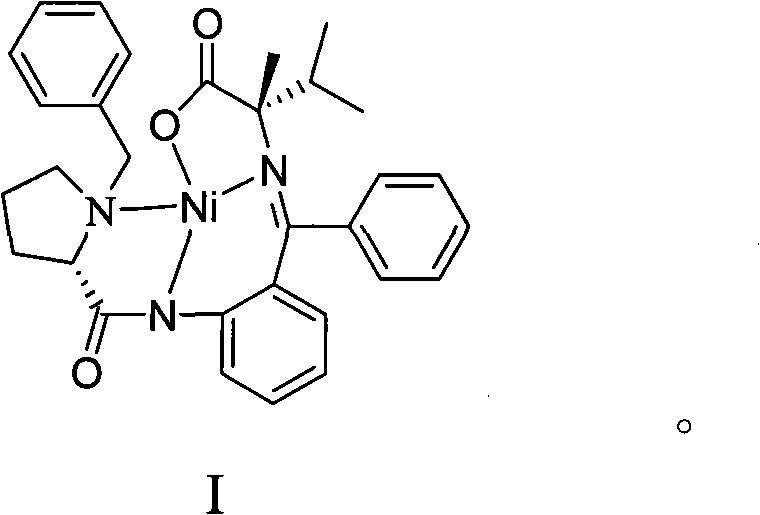
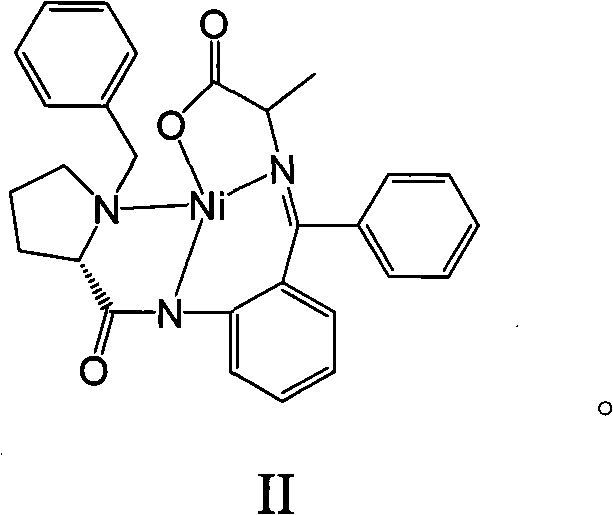
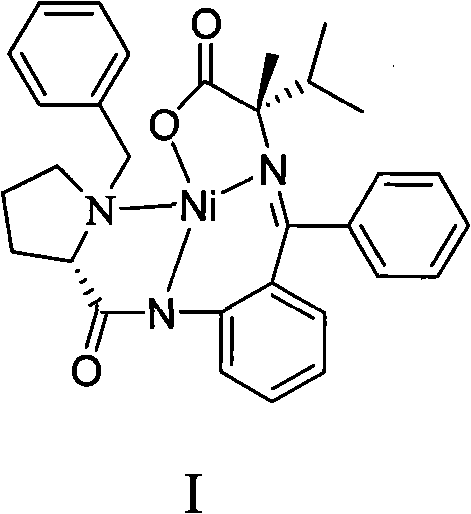
[0015] Dissolve 25.00 g (0.0486 mol) of compound II and 7.00 g (0.2916 mol) of sodium hydride in 75 mL of N,N-dimethylformamide, stir at room temperature for about 1 h, then add 18.37 g of isopropyl bromide (0.1458 mol), After the addition, the reaction solution was heated to 40-50°C, and stirred for about 2 hours, then an appropriate amount of distilled water was added dropwise to the reaction solution to precipitate crystals to obtain the amino acid Schiff base complex I with a yield of 71%. The measured values of elemental analysis of complex I: C, 67.01%; H, 6.03%; N, 7.65%; Ni, 10.61%; O, 8.70%. Theoretical: C, 67.17%; H, 6.00%; N, 7.58%; Ni, 10.59%; O, 8.66%. Complex I with CDCl 3 NMR of the solvent ( 1 H-NMR): δ: 8.04~8.10(m, ArH, 3H), 7.32~7.50(m, ArH, 7H), 7.10~7.13(m, ArH, 1H), 6.95~7.09(m, ArH, 1H) , 6.61~6.94(m, ArH, 2H), 4.54~4.57(m, -CH=, 1H), 2.42~3.58(m, -CH2-, 2H), 2.03~3.15(m, -CH 2 -,6H), 2.06(s,-CH 3 , 3H), 1.10~1.12(d, -CH 3 , 3H), 0.92~0.96(d, -CH...
Embodiment 2
[0017] Dissolve 20.00 g of amino acid Schiff base complex I in 25 mL of methanol, then add 20 mL of 6 mol / L hydrochloric acid, and heat to reflux. After cooling, remove prolyl-2-aminobenzophenone BPB by filtration, and then use ion exchange resin to remove nickel ions to obtain (S)-2-amino-2,3-dimethylbutyric acid with a yield of 90%. (S)-2-Amino-2,3-dimethylbutanoic acid measured elemental analysis values: C, 54.87%; H, 9.91%; N, 10.71%; O, 24.51%. Theoretical: C, 54.94%; H, 9.99%; N, 10.68%; O, 24.39%. (S)-2-Amino-2,3-dimethylbutanoic acid with D 2 O is the NMR of the solvent ( 1 H-NMR): δ: 2.14~2.21 (m, -CH=, 1H), 1.49 (s, -CH 3 , 3H), 0.99~1.05(m, -CH 3 , 6H). The results showed that the synthesized product was (S)-2-amino-2,3-dimethylbutanoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com