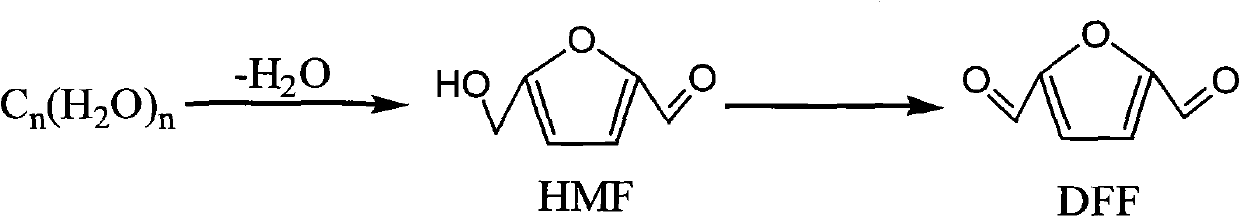
Method for catalysis-synthesizing 2,5-dicarbaldehyde by carbohydrate
A technology of carbohydrates and furandicarbaldehyde, applied in the direction of organic chemistry, can solve the problems of high energy consumption, high cost, low yield, etc., and achieve the effect of simple operation, short reaction time and less reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 0.08g glucose (0.45mmol), 1mLDMA, 0.007g CrCl 3 ·6H 2 O and 0.08g NaBr. After connecting the condenser tube, place it in an oil bath, raise the temperature of the oil bath to 100°C, keep the temperature constant, stir, and reflux for 6 hours to stop the reaction. The reaction system was naturally cooled to room temperature, and the CrCl was separated by centrifugation. 3 ·6H 2 O and NaBr, GC analysis indicated a 74% yield of HMF. Add 0.096mmolNaVO to the reaction solution 3 2H 2 O, air was bubbled through the mixture at a flow rate of 24 mL / min, the temperature of the oil bath was raised to 110° C., the temperature was kept constant, stirred, and the reaction was refluxed for 10 hours to stop the reaction. The reaction system was naturally cooled to room temperature, and NaVO was separated by centrifugation. 3 2H 2 O, GC analysis shows that the selectivity of DFF calculated with HMF is 70%, the yield is 69%, and the yield of DFF calculated with glucose is 51...
Embodiment 2-5
[0018] Prepare four reaction solutions containing HMF according to the method of Example 1, and add NaVO respectively 3 2H 2 O0.032mmol, 0.064mmol, 0.128mmol, 0.160mmol, and air was bubbled through the four mixtures at a flow rate of 24mL / min, and the oil bath was heated to 110°C, kept at constant temperature, stirred, and refluxed for 10 hours After stopping the reaction. The reaction system was naturally cooled to room temperature, and NaVO was separated by centrifugation. 3 2H 2 O. The reaction solution was detected by GC, and the results of the reaction under different amounts of catalysts are listed in Table 1.
[0019] Table 1
[0020] Implementation column
Embodiment 6-9
[0022] Prepare four reaction solutions containing HMF according to the method of Example 1, add 0.096mmol NaVO 3 2H 2 O, and the air is bubbled through the four mixed liquids at a flow rate of 8mL / min, 16mL / min, 32mL / min, and 40mL / min respectively, and the oil bath is heated to 110°C, constant temperature, stirring, and reflux reaction The reaction stopped after 10 hours. The reaction system was naturally cooled to room temperature, and NaVO was separated by centrifugation. 3 2H 2 O. The reaction solution was detected by GC, and the results obtained from the reaction at different air flow rates are listed in Table 2.
[0023] Table 2
[0024] Implementation column
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com