2-(2-pyridyl)-8-sulfonamidoquinoline derivative, synthesis method and application thereof
A sulfonamidoquinoline and pyridyl technology, which is applied in the field of 2--8-sulfonamidoquinoline derivatives, can solve the problem of the excitation wavelength entering visible light, etc., and achieves improved metal ion binding performance, easy availability of raw materials, and synthetic simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
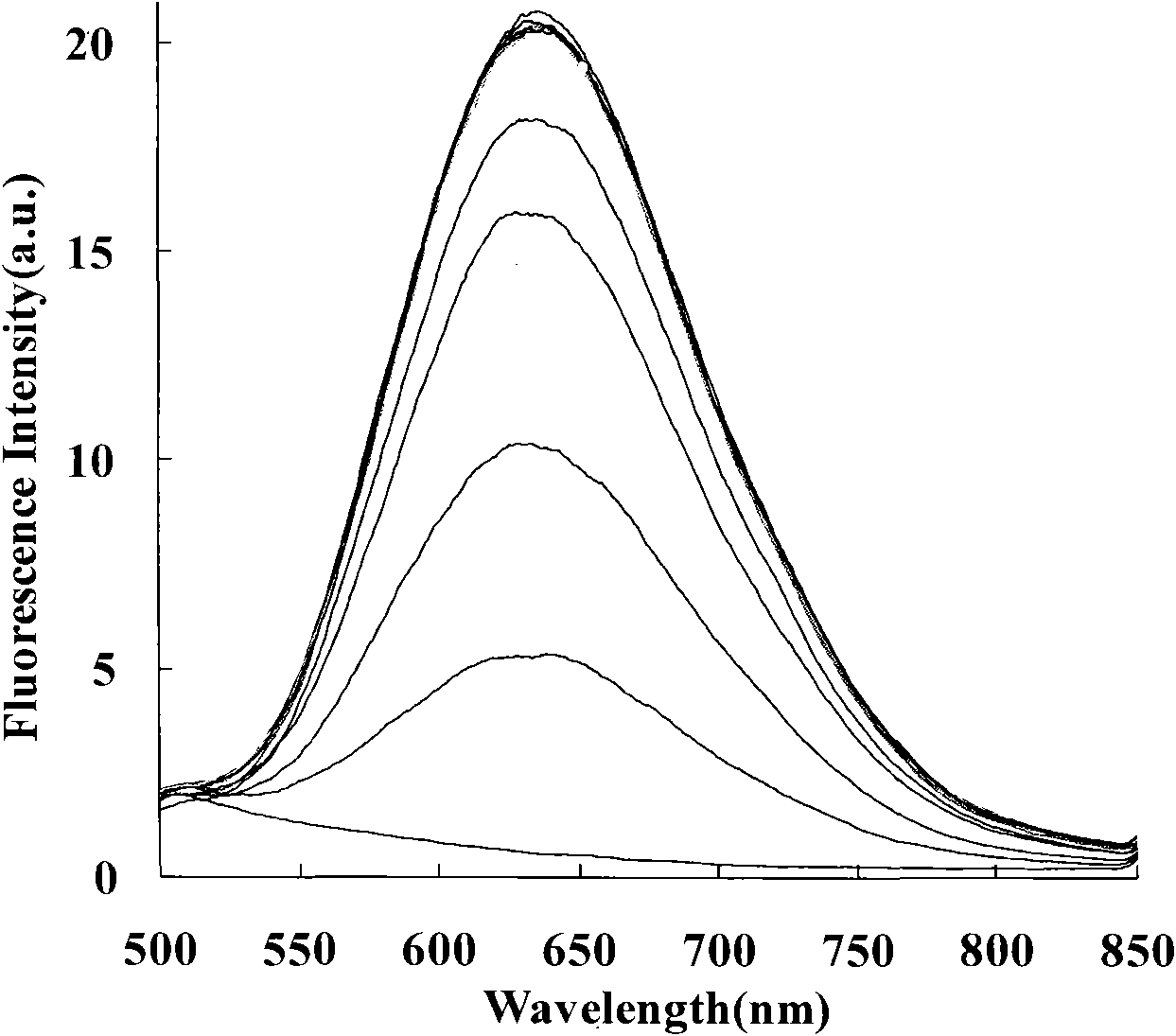
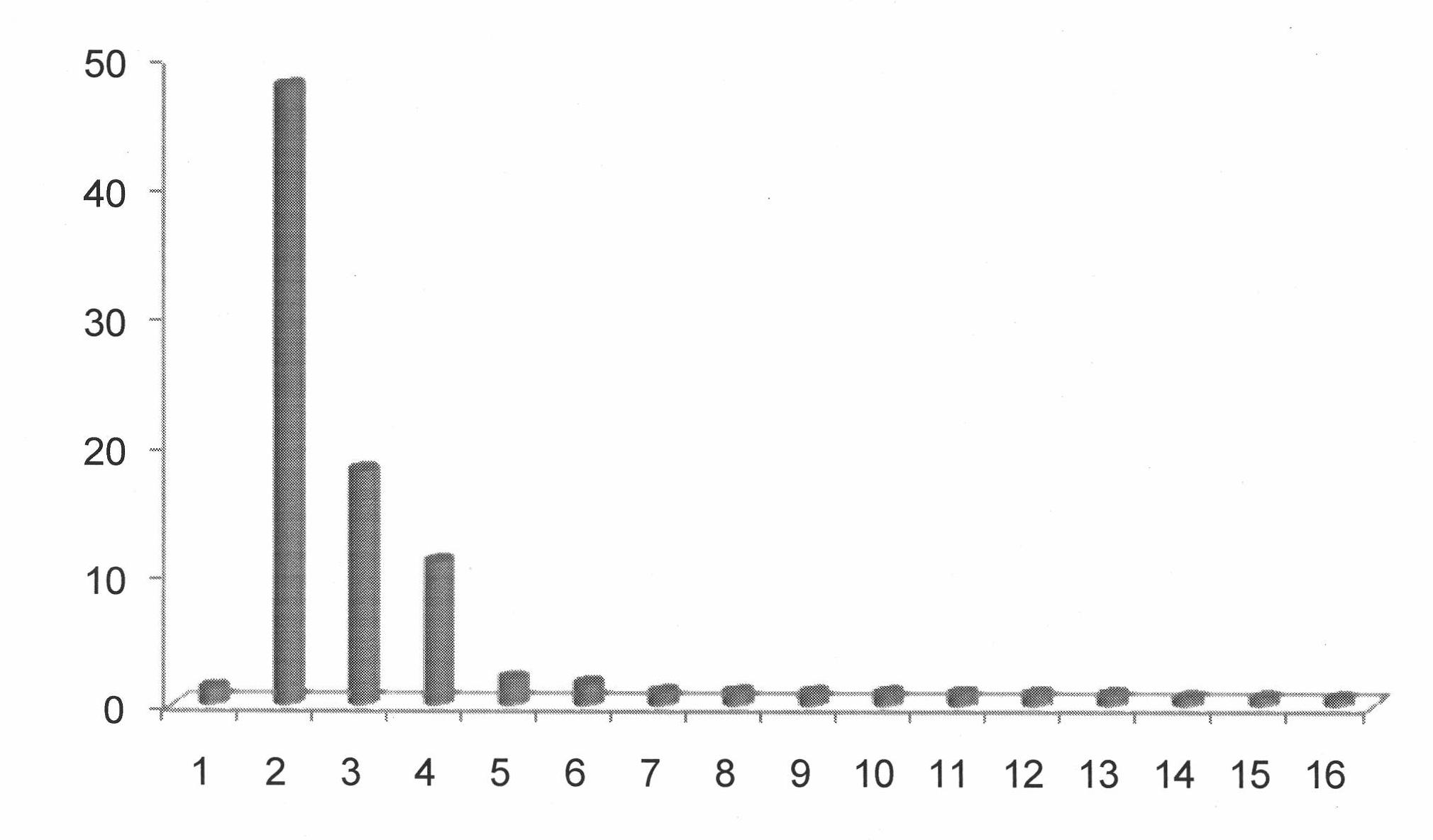
Image
Examples
Embodiment 1
[0021]
[0022] Dissolve 100mg (0.45mmol) of 2-pyridine-8-aminoquinoline and 357mg (4.52mmol) of pyridine in 10mL of dichloromethane, and add 105mg (0.54mmol) of p-toluenesulfonyl chloride in dichloromethane dropwise under ice-cooling 5mL solution, left at room temperature overnight. The reaction solution was spin-dried and separated by silica gel column chromatography to obtain 102 mg of the product with a yield of 62.1%. mp: 178.7-179.2°C.
[0023] 1 H-NMR (400MHz, CDCl 3 )δ( * 10 -6 ): 9.22(s, 1H), 8.75(d, J=4.0Hz, 1H), 8.60(d, J=5.6Hz, 1H), 8.55(d, J=8.0Hz, 1H), 8.22(d, J =8.4Hz, 1H), 7.95(t, J=8.0Hz, 1H), 7.84(m, 3H), 7.51(d, J=8.4Hz, 1H), 7.47(t, J=8.8Hz, 1H), 7.41(d, J=6.0Hz, 1H), 7.02(d, J=8.0Hz, 2H), 2.32(s, 3H).
Embodiment 2
[0025]
[0026] Operation method is referring to embodiment 1. The product yield is 50.8%. mp: 183.9-185.4°C.
[0027] 1 H-NMR (400MHz, CDCl 3 )δ( * 10 -6 ): 9.23(s, 1H), 8.76(d, J=4.4Hz, 1H), 8.60(d, J=8.8Hz, 1H), 8.52(d, J=8.0Hz, 1H), 8.24(d, J =8.8Hz, 1H), 7.95(t, J=7.6Hz, 1H), 7.84(d, J=8.4Hz, 2H), 7.82(d, J=8.8Hz, 1H), 7.54(d, J=8.4 Hz, 1H), 7.47(t, J=8.4Hz, 1H), 7.42(d, J=6.8Hz, 2H), 7.28(d, J=8.8Hz, 2H).
Embodiment 3
[0029]
[0030] Operation method is referring to embodiment 1. The product yield is 38.8%. mp: 202.4-203.3°C.
[0031] 1 H-NMR (400MHz, CDCl 3 )δ( * 10 -6 )): 9.22(s, 1H), 8.75(d, J=4.4Hz, 1H), 8.58(d, J=8.8Hz, 1H), 8.54(d, J=8.4Hz, 1H), 8.23(d, J=8.8Hz, 1H), 7.96(d, J=7.6Hz, 1H), 7.91(d, J=8.0Hz, 2H), 7.84(d, J=7.6Hz, 1H), 7.51(d, J= 8.4Hz, 1H), 7.46(d, J=7.6Hz, 1H), 7.41(m, 2H), 7.32(t, J=8.0Hz, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com