Application of jungermanniaceae D in preparing anti-tumor medicaments and anti-tumor multi-drug resistance reversing medicaments
An anti-tumor drug, the technology of orcin D, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as the application of orcin D that has not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Determination of the activity of orcin D to DNA topoisomerase I (TopoI) and topoisomerase II (TopoII)
[0020] (1) Formation of Leptosperin D:
[0021] Use an analytical balance to take 5 mg of phylloxin D, add 1179.24 μl of DMSO (cell culture grade) to obtain a 10 mM stock solution, and store it at -20°C (dilute to the required concentration with the medium before use).
[0022] (2) Extraction of DNA topoisomerase:
[0023] Collect K562 cells about 1×10 8 , wash twice with 4°C pre-cooled PBS, add lysate (20mM Tris, 1mM EGTA, 25mM KCI, 5mM MgCl 2 , 250mM sucrose, 0.5% NP40, pH 7.2) 1ml, lyse at 4°C for 10min, centrifuge at 6000rpm for 2min, add 50μl nuclear extract (20mM Tris, 1mM EGTA, 2mM EDTA, 2mM DTT, 400mM NaCl, pH 7.2) to the pellet and pipette evenly, Incubate on ice for 30 minutes, centrifuge at 14,000 rpm for 15 minutes, carefully aspirate the supernatant, measure the protein content with the Coomassie Brilliant Blue method, and store at -20°C aft...
Embodiment 2
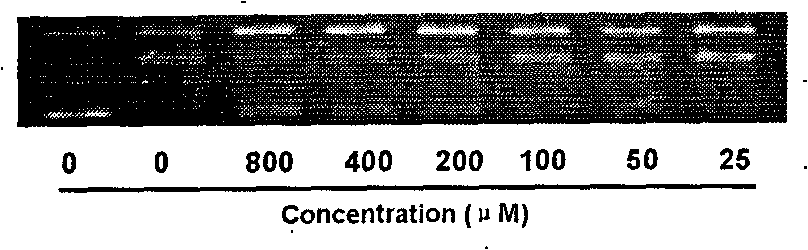
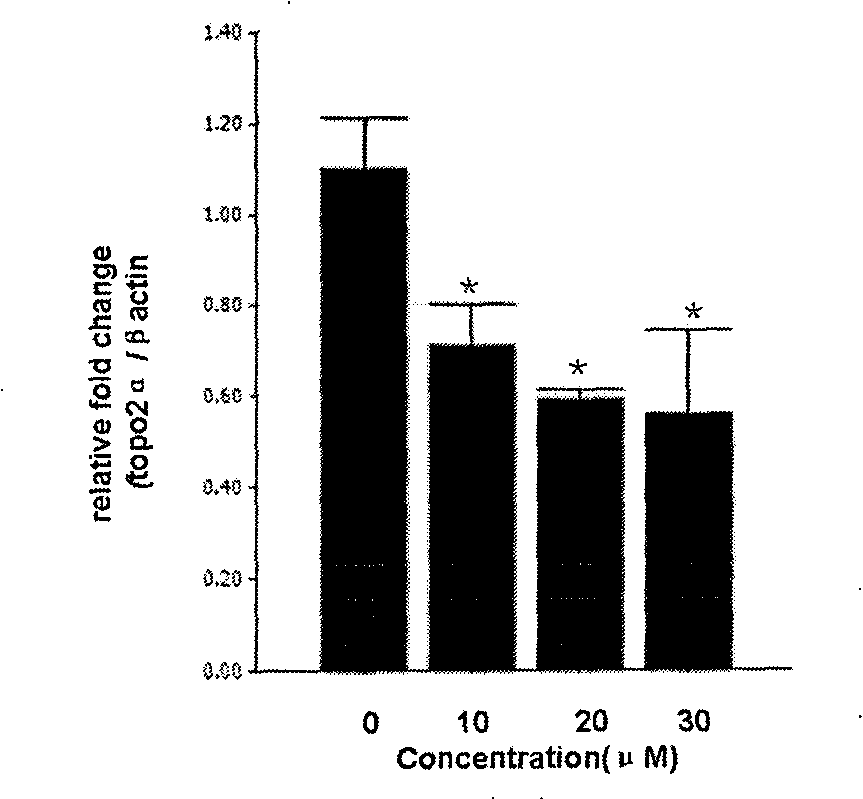
[0031] Example 2: Real-time Quantitative PCR Detection of the Inhibitory Effect of Leaforcin D on DNA Topoisomerase IIα Subtype (TopoIIα)
[0032] (1) RNA extraction
[0033] a. Cell treatment: Take K562 cells in the exponential growth phase and inoculate them in 6-well plates, the number of cells is 2×10 5 each / well, and at the same time, add different concentrations of leafletin D, and the final concentrations are 10, 20, and 30 μM, respectively. Set at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, the cells were collected into 1.5ml Ep tubes, washed twice with PBS, 3000rpm×5min.
[0034]b. Extraction of total RNA: Take the above precipitate and add 0.5ml Trizol to mix well, then add 100μl chloroform, mix up and down several times, room temperature for 5min. Centrifuge at 4°C, 12000G×15min. Then carefully pipette the upper aqueous phase containing total RNA into a new DEPC-treated Ep tube, and do not inhale any substance in the middle layer. 1ml Trizol ca...
Embodiment 3
[0054] Example 3: The growth inhibitory effect of lamellarin D on K562 / A02 cells
[0055] Method: MTT method: take chronic myelogenous leukemia drug-resistant cell line K562 / A02, 2×10 4 Cells per well were seeded in a 96-well plate, and 0.4% DMSO and different concentrations of orcin D were added respectively. After culturing for 24, 48 and 72 hours, 20 μl of 5 mg / ml MTT was added to each well and continued to incubate for 4 hours. 200 μl of DMSO was shaken slightly to completely dissolve the formed formazan for color development, and the OD value was measured at a wavelength of 570 nm with a Bio-Rad Model 550 Microplate Reader. The experiment was carried out three times under different culture time conditions, and the average value was calculated.
[0056] Result: if Figure 4 As shown, it can be seen from the figure that the 24, 48 and 72 h cultured under the condition of 2 to 10 μg / ml, respectively, has no obvious inhibitory effect on the K562 / A02 cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com