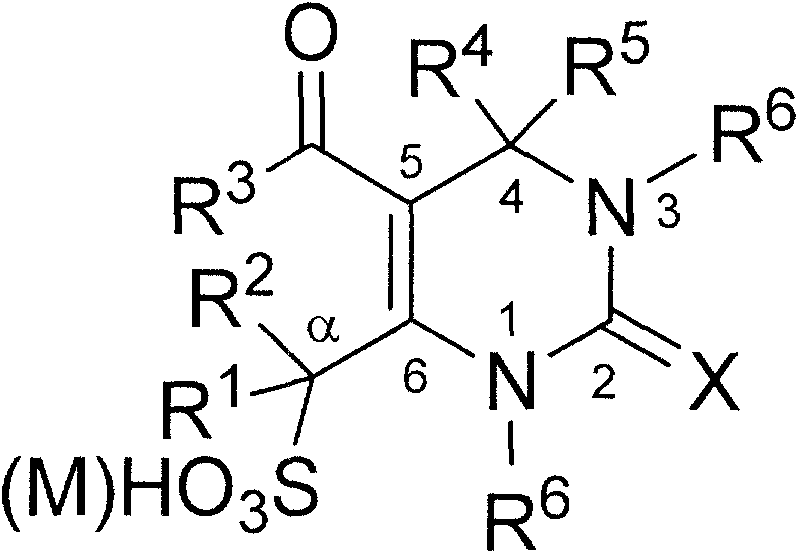
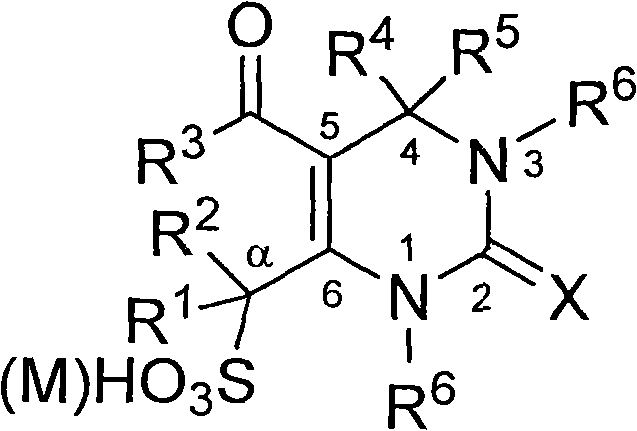
Dihydro pyrimidone sulfoacid as well as salt compound and preparation method thereof
A technology of dihydropyrimidinone sulfonate and dihydropyrimidinone sulfonic acid is applied in the field of synthesizing heterocyclic compounds, and can solve the problem of low water solubility, influence on drug availability and effective time, dihydropyrimidinone/thione class Problems such as low water solubility and fat solubility of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 5-ethoxycarbonyl-4-phenyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one (2.6 g, 10.0 mmol), 20 mL of dichloromethane to the dry reactor, Cool in an ice-salt bath, stir and add chlorosulfonic acid (2.0mL, 30.0mmol), heat up to 40°C after addition, stir, react for 30 minutes, cool, stand still, separate the upper layer of dichloromethane, wash the lower layer with acetonitrile, and precipitate The white precipitate was filtered and recrystallized with ethanol-water to obtain 5-ethoxycarbonyl-2-oxo-4-phenyl-3,4-dihydropyrimidin-2(1H)-one-6-methylsulfonic acid, the product 2.7 g, 79% yield, m.p. 274°C (decomp.).
[0020] Molecular formula is C 14 h 16 N 2 o 6 S, ESI-MS m / z (%): 339.35 (M - , 100). Anal. Calc.: C, 49.40; H, 4.74; N, 8.23; S, 9.42%; found: C, 49.24; H, 4.68; N, 8.15;
[0021] IR (KBr, cm -1 ): v3244, 3105, 2982, 1699, 1644, 1456, 1229, 1171, 1096.
[0022] 1 H NMR (400MHz, D 2 O): δ1.08(t, 3H, J=7.0Hz, CH 3 ), 4.00 (q, 2H, J=7.0Hz, CH 3 -Et), 4.43(q...
Embodiment 2
[0024] To the dry reactor was added 5-ethoxycarbonyl-4-(4'-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (2.9 g, 10.0 mmol) , 20mL of dichloromethane, cooled in an ice-salt bath, stirred and added dropwise chlorosulfonic acid (2.0mL, 30.0mmol), heated to 40°C after the addition, stirred, reacted for 30 minutes, cooled, stood still, separated the upper layer of dichloromethane, and the lower layer The substance was washed with acetonitrile, and a white precipitate was precipitated, filtered, eluted with a mixture of ethyl acetate and methanol (2:1), and column chromatography gave 5-ethoxycarbonyl-2-oxo-4-(4'-chlorophenyl )-3,4-dihydropyrimidin-2(1H)-one-6-methanesulfonic acid product 2.5g, yield 67%, m.p.210°C.
[0025] Molecular formula is C 14 h 15 ClN 2 o 6 S, ESI-MS m / z (%): 373.16 (M - , 100).Anal.Calc.: C, 44.86; H, 4.03; Cl, 9.46; N, 7.47; S, 8.56%; found: C, 44.62; 8.43%.
[0026] IR (KBr, cm -1 ): v3273, 3011, 2939, 1710, 1659, 1232, 1181, 1127, 1042....
Embodiment 3
[0030] Add 5-ethoxycarbonyl-4-methyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one (1.98g, 10mmol) to the dry reactor, 20mL dichloromethane, ice Cool in a salt bath, stir and add chlorosulfonic acid (2.0mL, 30mmol) dropwise, heat up to 40°C after addition, stir, react for 30 minutes, cool, let stand, separate the upper layer of dichloromethane, wash the lower layer with ether, and precipitate white The solid was filtered by suction, eluted with a mixture of ethyl acetate and methanol (2:1), and column chromatography gave 5-ethoxycarbonyl-2-oxo-4-methyl-3,4-dihydropyrimidine-2 ( 1H)-Keto-6-methylsulfonic acid product 2.5g, yield 90%, m.p.207°C.
[0031] Molecular formula is C 9 h 14 N 2 o 6 S, ESI-MS m / z (%): 277.34 (M - , 100). Anal. Calc.: C, 38.84; H, 5.07; N, 10.07; S, 11.52%; found: C, 38.75;
[0032] IR (KBr, cm -1 ): v3298, 3025, 2936, 1716, 1575, 1233, 1167, 1139, 1031.
[0033] 1 H NMR (400MHz, D 2 O): δ1.15(d, 3H, J=6.40Hz, CH 3 -Pyr), 1.19(t, 3H, J=6.95Hz, CH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com