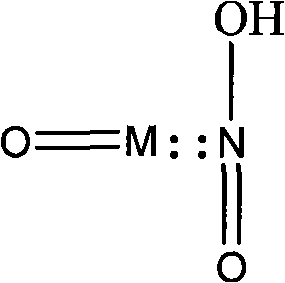
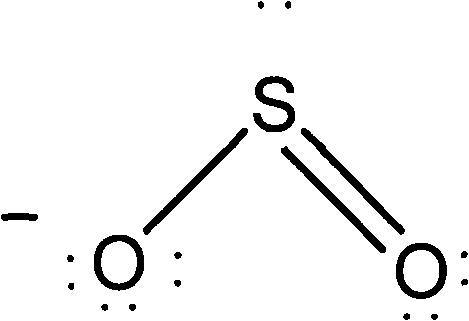
Method for removing acid gas from waste gas
A technology for acid gas and waste gas, applied in the fields of SOx, Hg and NOx, which can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A glass column with a height of 400mm and a diameter of 30mm was heated to 80°C using an external electric heater. The glass column was filled with a glass ring with a height of 4 mm and a diameter of 4 mm and a mixture of water, magnesium oxide and sulfoxide in a weight ratio of 40%:10%:50%.
[0073] Continuously feed air (0.33 L / min) diluted SO from the bottom of the column 2 (850 ppm; 0.15 L / min) air flow with a contact time of 5.0 seconds with the wash mixture. The experiment lasted for 420 minutes and no SO was emitted 2 , thus proving that all SO 2 are collected.
Embodiment 2
[0075] A glass column with a height of 400mm and a diameter of 30mm was heated to 80°C using an external electric heater. The glass column was filled with a glass ring with a height of 4 mm and a diameter of 4 mm and a mixture of water, magnesium oxide and sulfoxide in a weight ratio of 65%:5%:30%.
[0076] Diluted SO was continuously fed with air (0.33 L / min) through the bottom of the column. 2 (850 ppm; 0.15 L / min) air flow with a contact time of 5.0 seconds with the wash mixture. The experiment lasted 240 minutes, no SO was emitted 2 , thus proving that all SO 2 are collected.
Embodiment 3
[0078] A glass column with a height of 400mm and a diameter of 30mm was heated to 80°C using an external electric heater. The glass column was filled with a glass ring with a height of 4 mm and a diameter of 4 mm and a mixture of water, potassium hydroxide and sulfoxide in a weight ratio of 40%:10%:50%.
[0079] Continuously feed air (0.33 L / min) diluted SO from the bottom of the column 2 (840 ppm; 0.15 L / min) air flow with a contact time of 5.0 seconds with the wash mixture. The experiment lasted for 420 minutes and no SO was emitted 2 , thus proving that all SO 2 are collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com