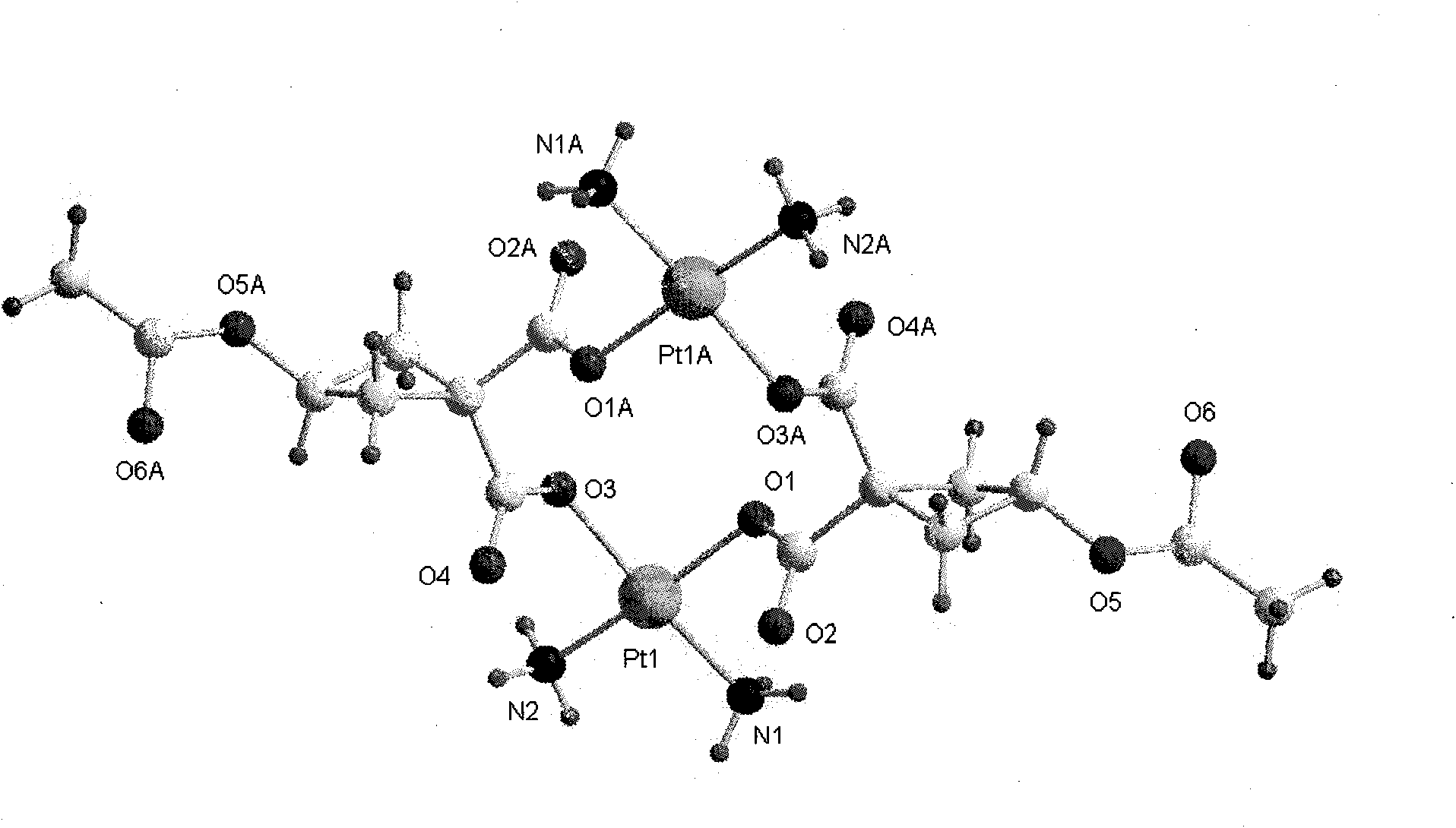
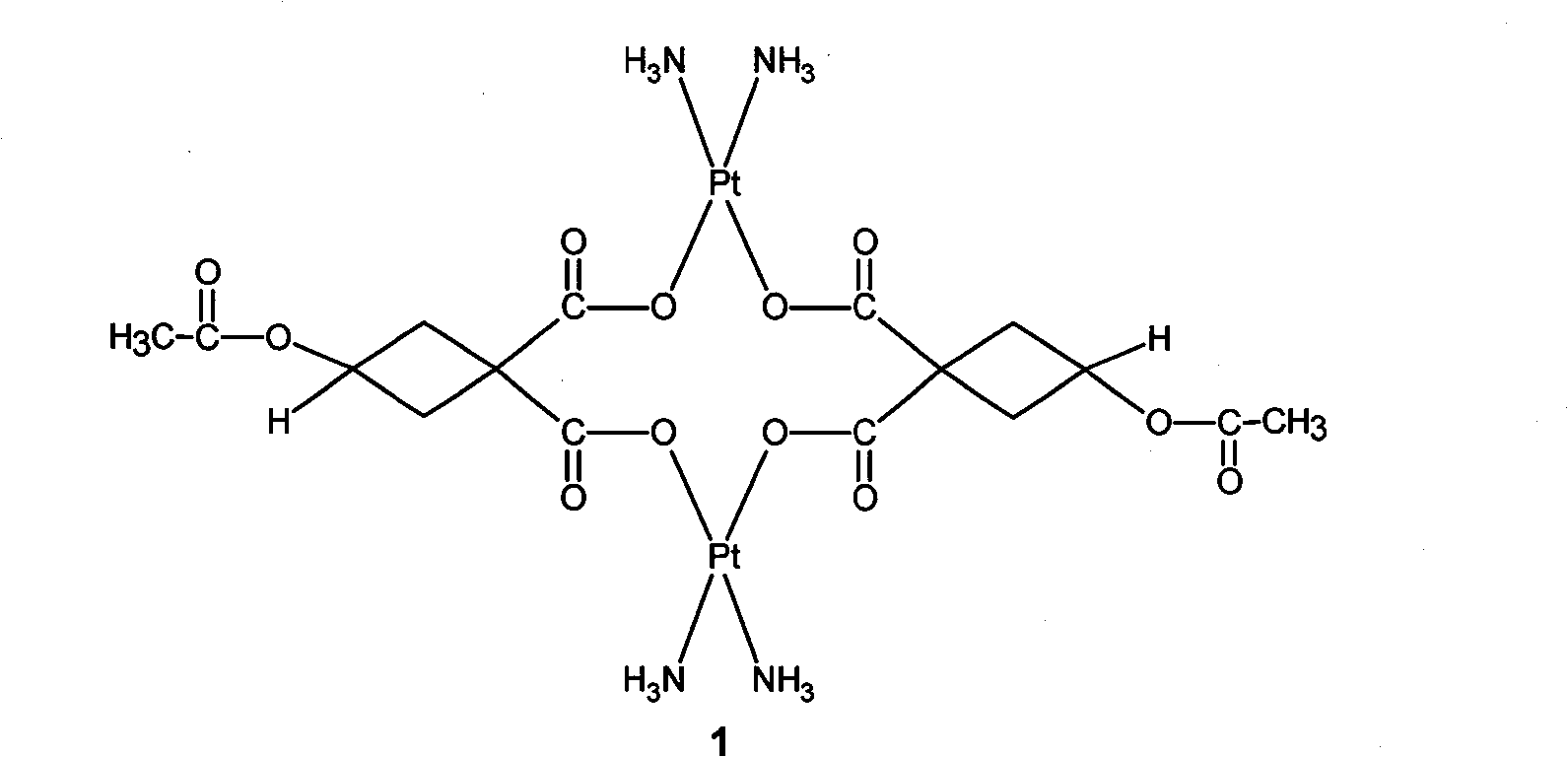
Water-soluble carboxyl-bridge dicaryon Pt (II) anti-tumor complex
An anti-tumor, water-soluble technology, applied in the direction of anti-tumor drugs, active ingredients of heavy metal compounds, compounds containing Group 8/9/10/18 elements of the periodic table, etc. Big side effects etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] (1) Preparation of 3-acetoxy-1,1-silver cyclobutane dicarboxylate
[0015] Firstly, 3-hydroxy-1,1-cyclobutane dicarboxylic acid (mp 151-152° C.) was prepared according to the method reported in the literature [Inorganica Chimica Acta, 2004, 357, 4452-4466]. Take 20g of 3-hydroxy-1,1-cyclobutanedicarboxylic acid, dissolve it in 200ml of acetone, add 33g of freshly distilled acetyl chloride, stir at 50°C for 4h, evaporate the solvent to obtain a crude product, recrystallize in isopropyl ether to get White crystals were dried at 60°C to obtain 15.5 g of 3-acetoxy-1,1-cyclobutanedicarboxylic acid, mp: 128-129°C, yield 60%.
[0016] Take 10g of 3-acetoxy-1,1-cyclobutanedicarboxylic acid, dissolve it in 100ml of water, and use 1mol / L NaHCO 3 Regulate PH=5-6, add 104mmol, 100ml AgNO3 (excessive 5%), obtain 3-acetoxy-1,1-silver precipitate of cyclobutane dicarboxylate, collect by filtration, after washing with water, ethanol, in 60-70 After vacuum drying at °C for 4 hours, 19...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com