Compound and application thereof in preparing drug for curing viral respiratory infection
The technology of a compound and a drug is applied in the preparation of a drug for treating respiratory virus infection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
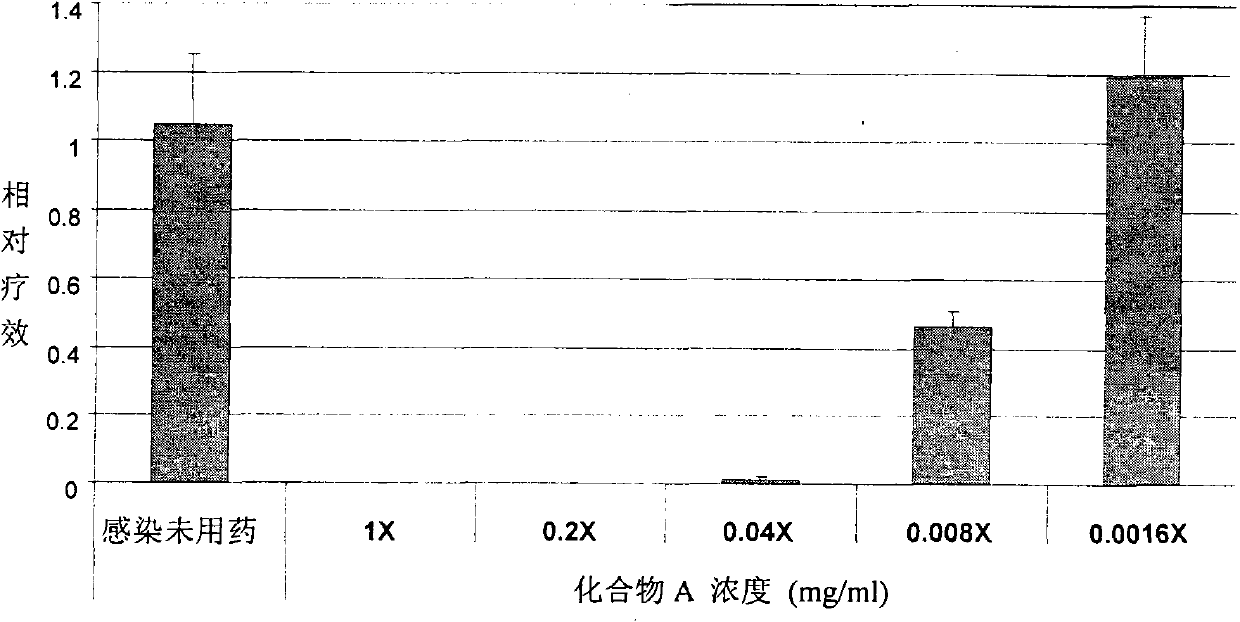
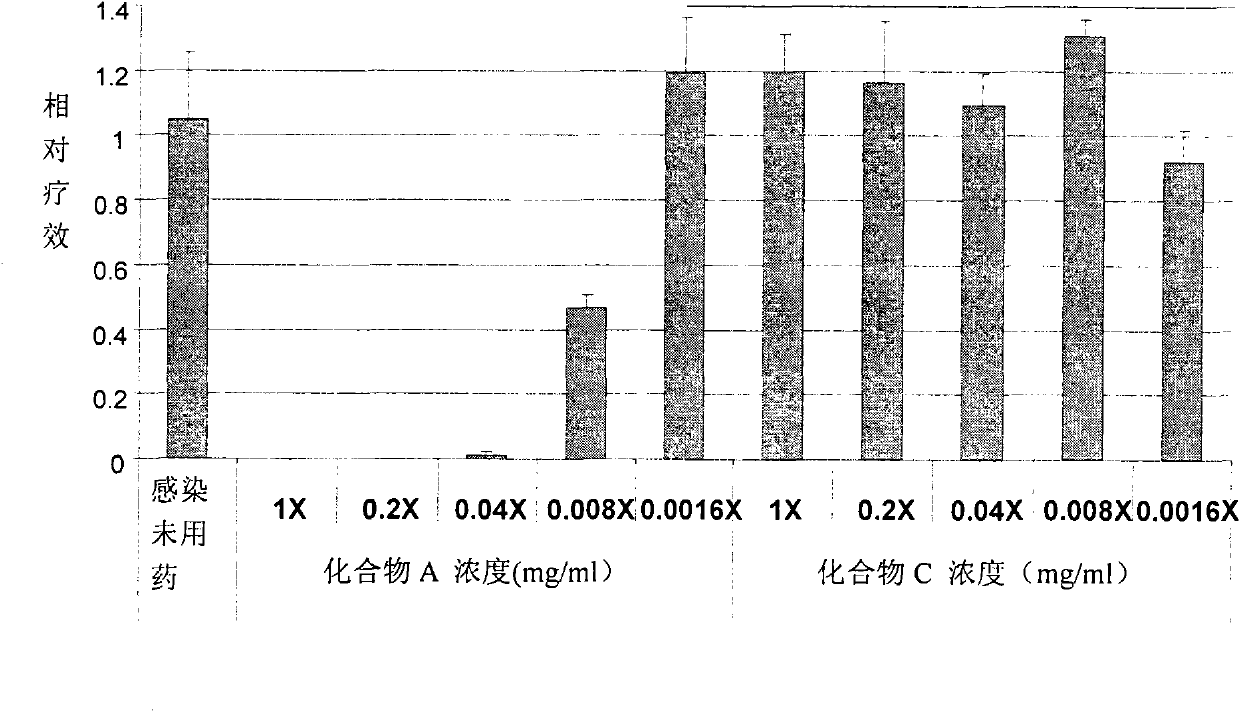
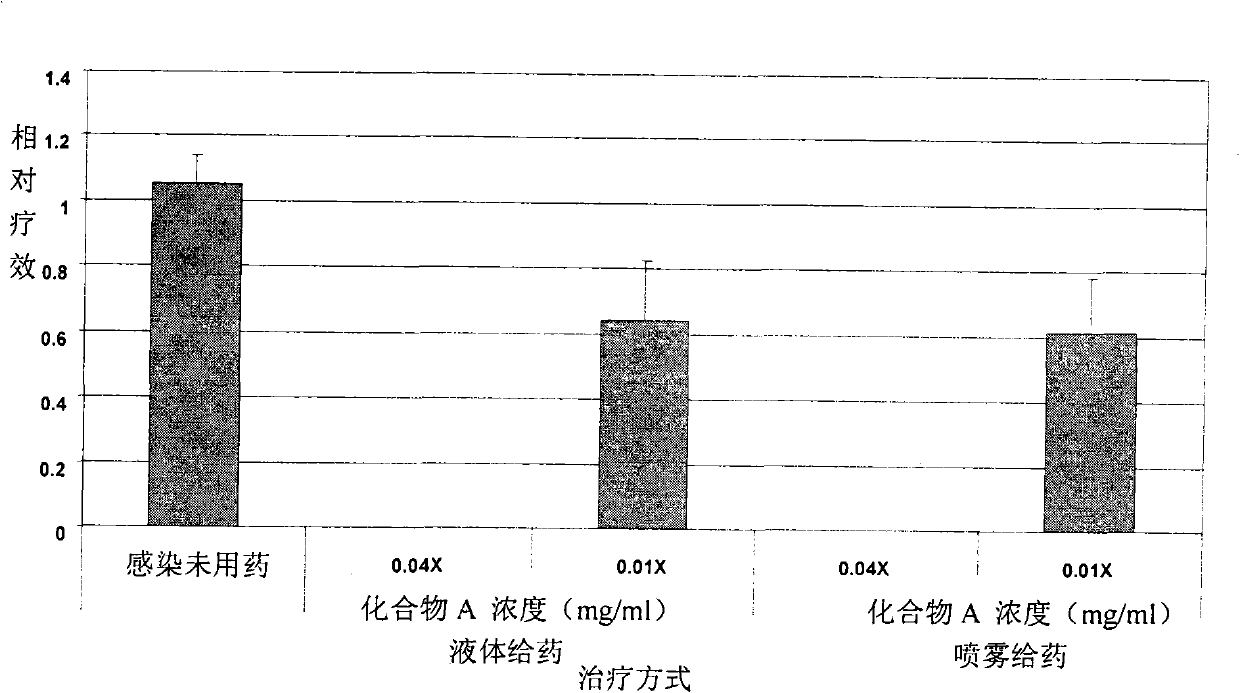
[0064]Human airway epithelial tissue cells were planted on a support mesh made of nylon and allowed to grow at the liquid-air interface. It was cultivated as a modified Ex-Cell Vero serum-free medium (purchased from Invitrogen). PR8 influenza virus was infected on the surface of respiratory epithelial tissue for 4 hours, and then directly administered (spray administration) through the surface of the tissue or added Tyloxapol (compound A) or neutral control (compound C) and positive control (Tamiflu ) (liquid administration). according to Figure 1-7 After treatment for the indicated time, the samples were collected, RNA was isolated and the virus was quantified by quantitative RT-PCR method. At the same time, the culture fluid was collected to detect LDH for toxicity analysis. see results Figure 1-7 .
Embodiment 2
[0066] After the mouse is lightly anesthetized, it is used to erect the mouse with its nose up, and then use a micropipette to transfer the PR8 virus
[0067] (2000pfu / 300μl in PBS) was dripped into the external nostril of the mouse to inhale, and the mouse was kept upright for 5 seconds to ensure that the mouse sucked all the solution into the trachea. Then use a warming lamp to keep the mouse warm for about 20 minutes or until the mouse resumes its activities. After the mouse is infected with the influenza virus for 24 hours, use the above method to drip 50 μl 4mg / mL Tyloxapol solution into the mouse through the nasal passage. The dose of Tamiflu is 10mg / kg, the untreated group and the PBS nutrient solution group were used as controls (except the untreated group, each group was given an equal volume of the corresponding solution twice a day), and the lungs of the mice were taken after 96 hours of infection, and the RNA was isolated and analyzed by quantitative RT-PCR. Method...
Embodiment 3
[0069] Dissolve Calixarenes in 0.9% sodium chloride solution, adjust the pH to about 7.0 with NaOH or HCl, and use 0.9% sodium chloride solution to make the final concentration 4mg / ml. After the mice were lightly anesthetized, the mice were erected with their noses up, and then the PR8 virus (2000pfu / 300μl in PBS) was dripped into the external nostrils of the mice with a micropipette for inhalation, and the mice were kept upright for 5 seconds while ensuring that the mouse draws all the solution into the trachea. Then use a warming lamp to keep the mice warm for about 20 minutes or until the mice resume their activities. 24 hours after the mice are infected with the influenza virus, use the above method to instill 50 μl of 4mg / ml Calixarenes (calixarenes) solution into the mice through the nasal passages , the dose of Tamiflu is 10mg / kg body weight, the infection untreated group and the PBS nutrient solution group are the controls (except the untreated group, each group is giv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com