Tertiary amine derivatives and their uses for treating a viral infection
A technology of compounds and medicinal salts, which is applied in the medical field and can solve problems such as restrictions on the application of antiviral drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0220] According to a further specific embodiment of the present invention, B represents:
[0221]
[0222] in:
[0223] -n is 0 or 1,
[0224] -R 2’ selected from hydrogen, oxygen, (C 1 -C 3 ) Alkyl and (C 1 -C 3 ) alkoxy, the (C 1 -C 3 ) Alkyl and (C 1 -C 3 ) alkoxy is optionally substituted by at least one halogen or hydroxyl,
[0225] -R 3’ selected from hydrogen, halogen, hydroxyl, cyano, (C 1 -C 3 ) Alkyl and (C 1 -C 3 ) alkoxy, the (C 1 -C 3 ) Alkyl and (C 1 -C 3 ) alkoxy is optionally substituted by at least one halogen or hydroxy, and
[0226] -R 4’ is hydrogen or -CONHR 5’ group, where R 5 ’ is hydrogen or (C 1 -C 3 )alkyl.
[0227] Preferably, B means:
[0228]
[0229] in:
[0230] -n is 0 or 1, preferably 1,
[0231] -R 2’ selected from hydrogen, oxygen and (C 1 -C 3 ) alkyl, preferably methyl, said (C 1 -C 3 ) alkyl is optionally substituted by at least one hydroxy group, R 2’ More preferably hydrogen,
[0232] -R 3’ is hyd...
Embodiment A
[0311] Example A - Chemistry
[0312] General synthetic route:
[0313] 1. Benzylic and aromatic substitution
[0314] -Route A:
[0315] By 1) using K 2 CO 3 (potassium carbonate) and CH 3 CN (acetonitrile) for benzylic or aromatic substitution followed by 2) using a) LiOH (lithium hydroxide) and b) NH 3 (Ammonia) and HATU (azabenzotriazole tetramethyluronium hexafluorophosphate) form an amide to prepare B as A compound of formula (I).
[0316] -Route B:
[0317] By 1) using K 2 CO 3 (potassium carbonate), CH 3 CN (acetonitrile) and Wilkinson catalyst (RhCl(PPh 3 ) 3 ) for a ruthenium-catalyzed synthesis followed by 2) using NaBH 4 (sodium borohydride), AcOH (acetic acid) and MeOH (methanol) or NaBH (OAc) 3 (sodium triacetoxy borohydride), and DCE (dichloroethane) carry out benzylic or aromatic substitution, preparation B is A compound of formula (I).
[0318] 2. Aliphatic and Heterocycloalkylamides
Embodiment B
[0320] Example B - Biology
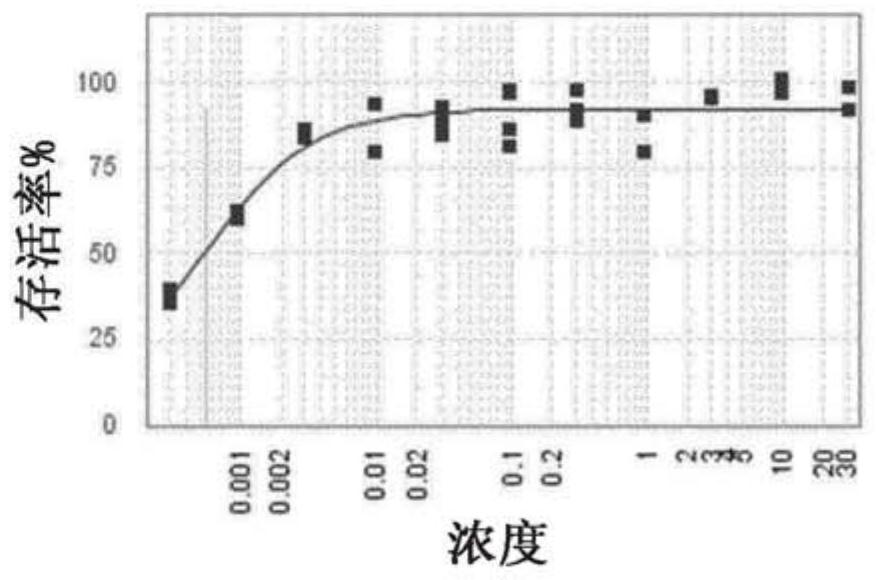
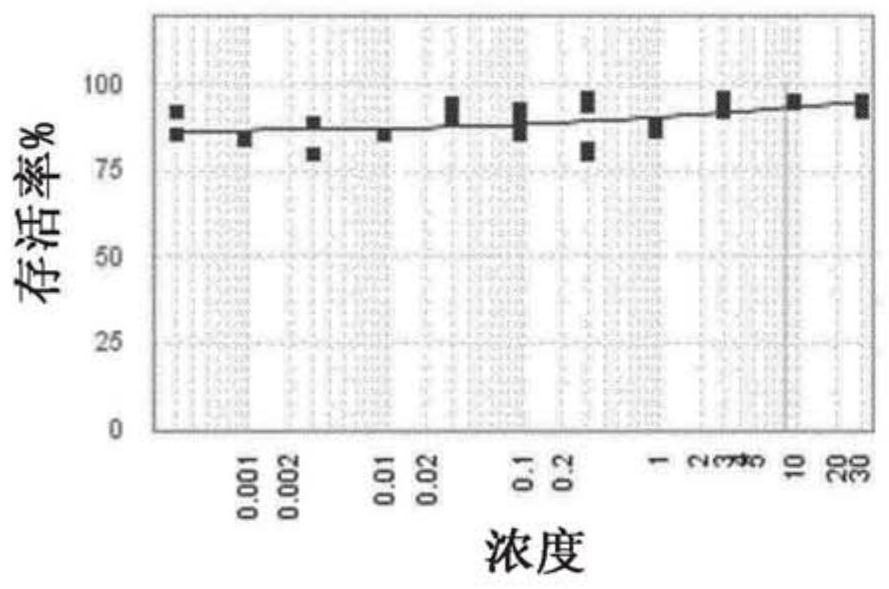
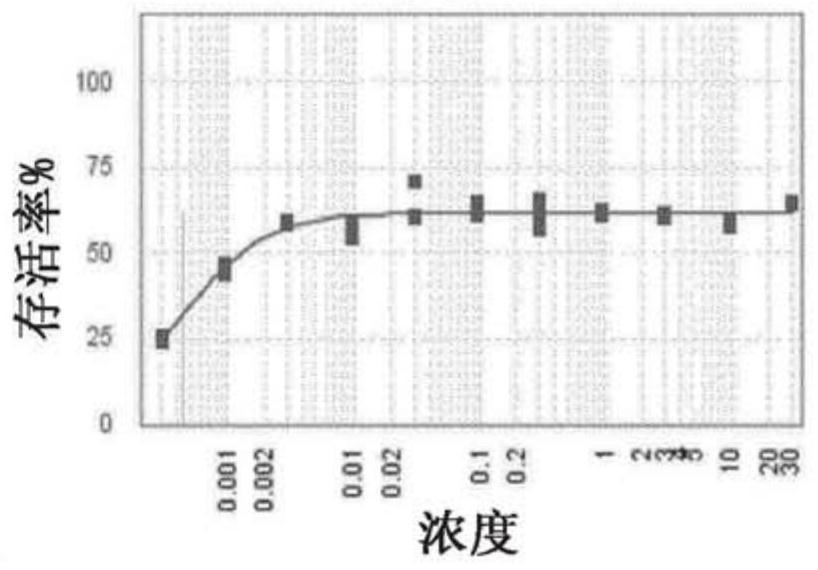
[0321] antiviral effect
[0322] Materials & Methods
[0323]
[0324] Cytopathic Effect (CPE) Determination Experimental Procedure:
[0325] RSV titer: 6.9x10 6 pfu / ml
[0326] MOI = Pfu / number of cells used for infection
[0327] 1) Seed HEp-2 cells at 5000 cells / well in 25 μl assay medium in a 384-well plate at 37°C in 5% CO 2 Incubate for 24 hours. Every well was seeded with cells except the first row which contained medium only (low control).
[0328] 2) Compounds were dispensed using an Echo550 liquid handler. All compounds were used at 10 mM stock concentration. The positive control ribavirin was included as a control compound in all assays.
[0329] 3) Cells are expected to double every 24 hours.
[0330] 4) The next day, cells were infected with RSV MOI 0.5, and 5 μl of virus diluted in assay medium was added.
[0331] 5) Treat uninfected cells (high control) with 5 μl of assay medium.
[0332] 6) Centrifuge the pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com