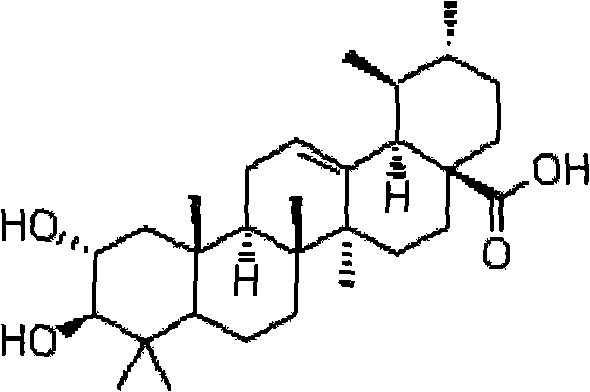
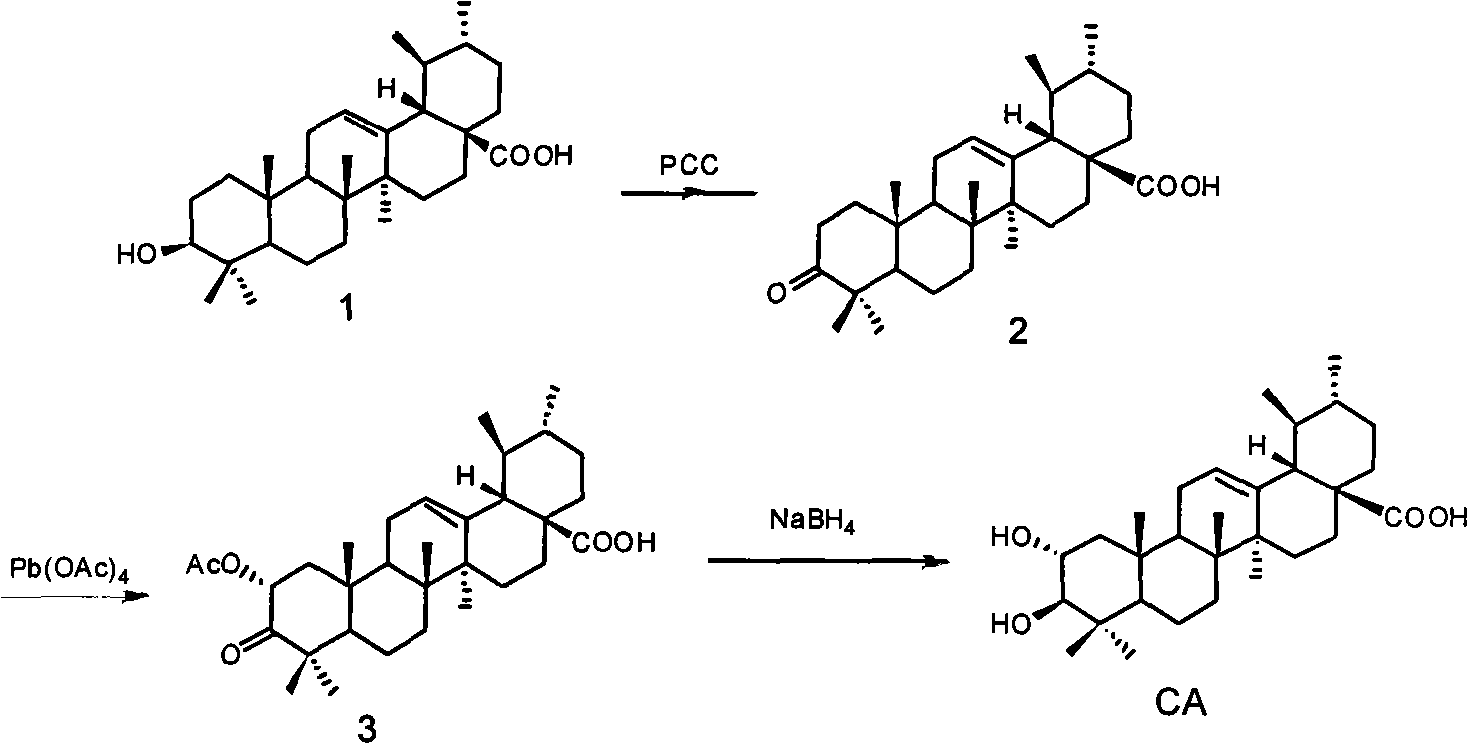
Preparation method of corosolic acid
A technology of corosolic acid and lead tetraacetate, applied in the field of medicine, can solve the problems of high investment cost, low yield, complicated production steps, etc., and achieve the effect of reducing the processing process, reducing the cost and shortening the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described below in conjunction with the examples, but not limited to the examples.
[0027] A preparation method of corosolic acid is provided:
[0028] Step 1 in the method: the preparation of 3-carbonyl ursolic acid
[0029] Add 20 grams of ursolic acid and 1000 ml of dichloromethane into the reaction flask, and add 10 grams of freshly prepared PCC in batches. TLC tracked until the raw materials disappeared, the reaction solution was filtered through diatomaceous earth, the filtrate was evaporated to dryness under reduced pressure, and recrystallized with ethanol to obtain 17.2 g of white crystals of 3-carbonyl ursolic acid with a yield of 88%.
[0030] Step 2 in the method: the preparation of 2-acetoxy-3-carbonyl ursolic acid
[0031] Take 15 grams of 3-carbonyl ursolic acid obtained in step 1 and dissolve it in 100 ml of benzene, add 29 grams of lead tetraacetate, and react in the dark at 60°C for 24 hours. After the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com