6-methy salicylic acid synthetase transformed by genetic engineering and combinatorial biosynthesis of spirocyclic acetoacetic acid lactone antiboitic
A spiro-acetoacetate and antibiotic technology, applied in the field of biotechnology engineering, can solve problems such as limited production prospects
Active Publication Date: 2010-08-25
国科新研国际技术转移有限公司
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
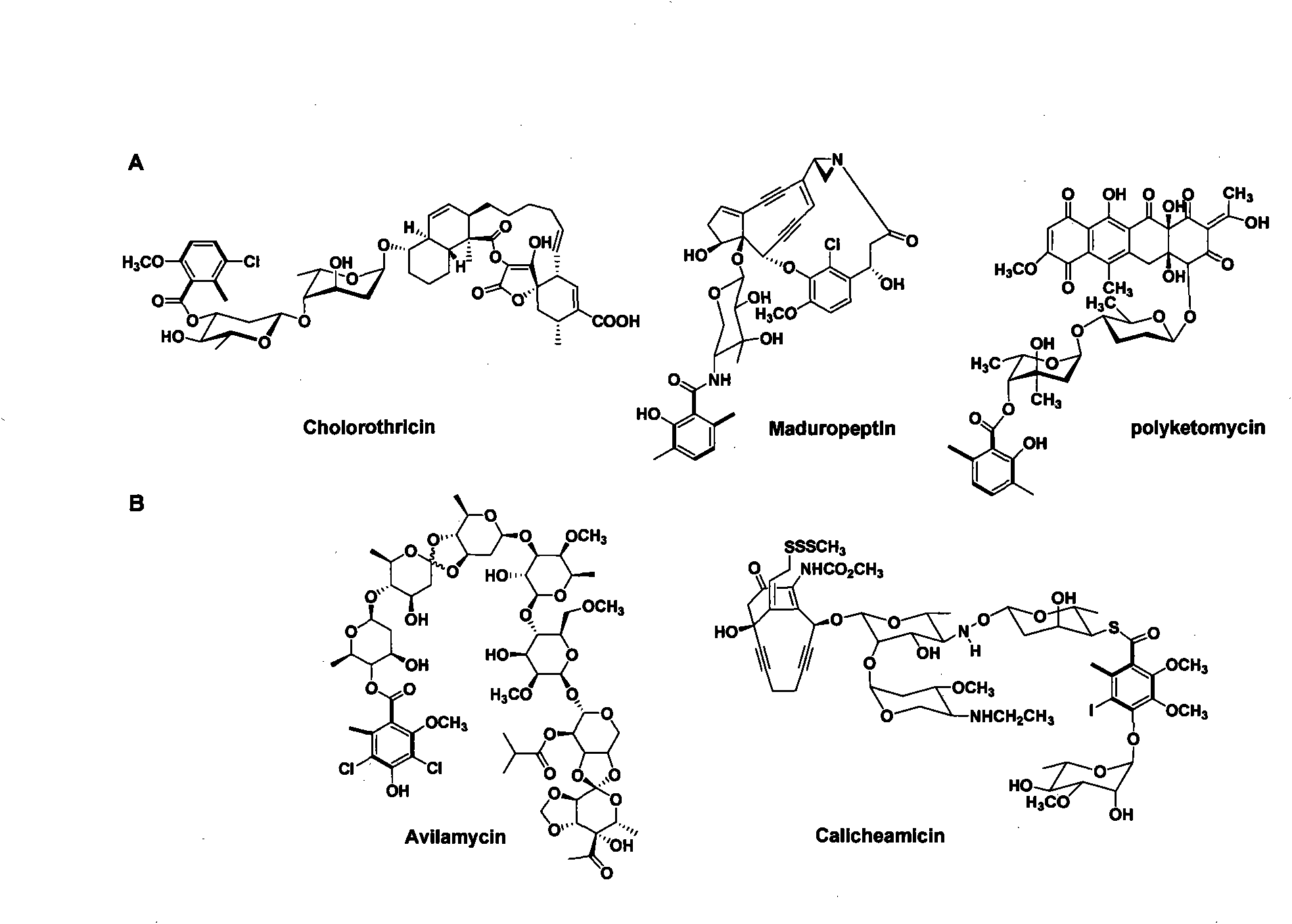
However, its complex chemical structure has brought great challenges to chemical synthesis and structure derivation. Due to the excessive chiral centers and concentrated functional groups, chemical synthesis has very limited prospects for the production of CHL and its analogues. An important supplement, the development of combinatorial biosynthesis technology provides a new method for the acquisition of complex natural products and their analogs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
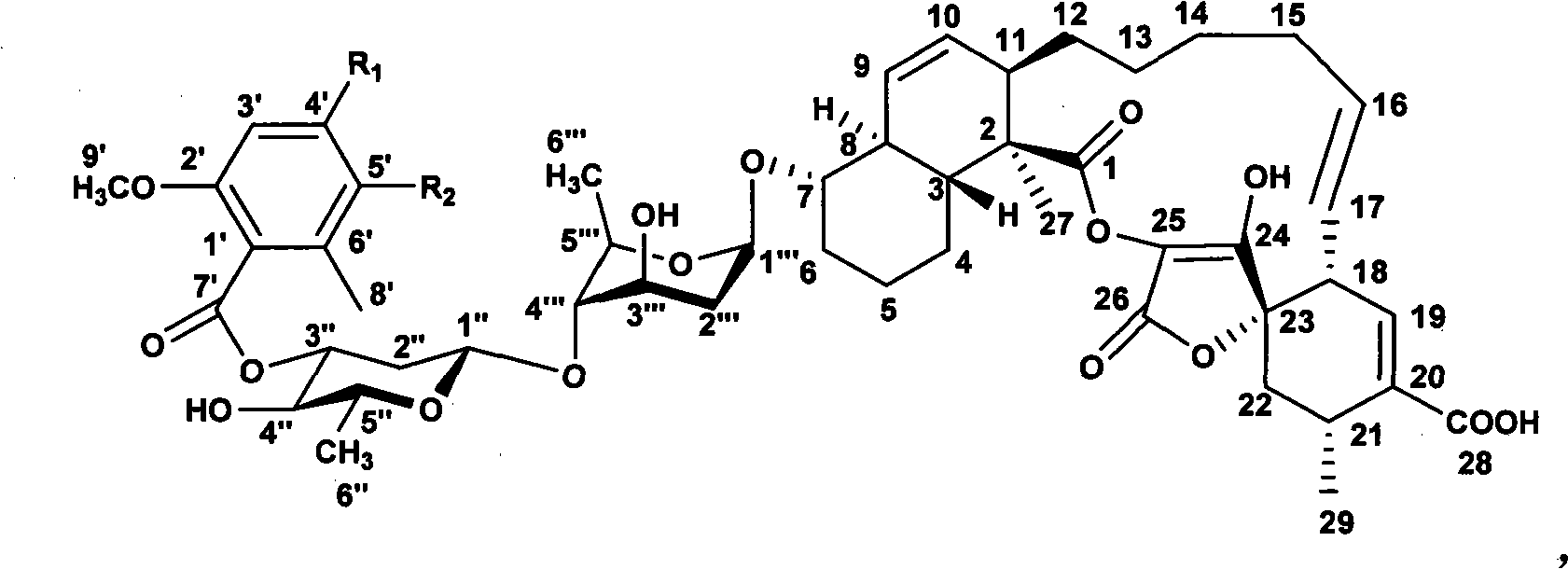
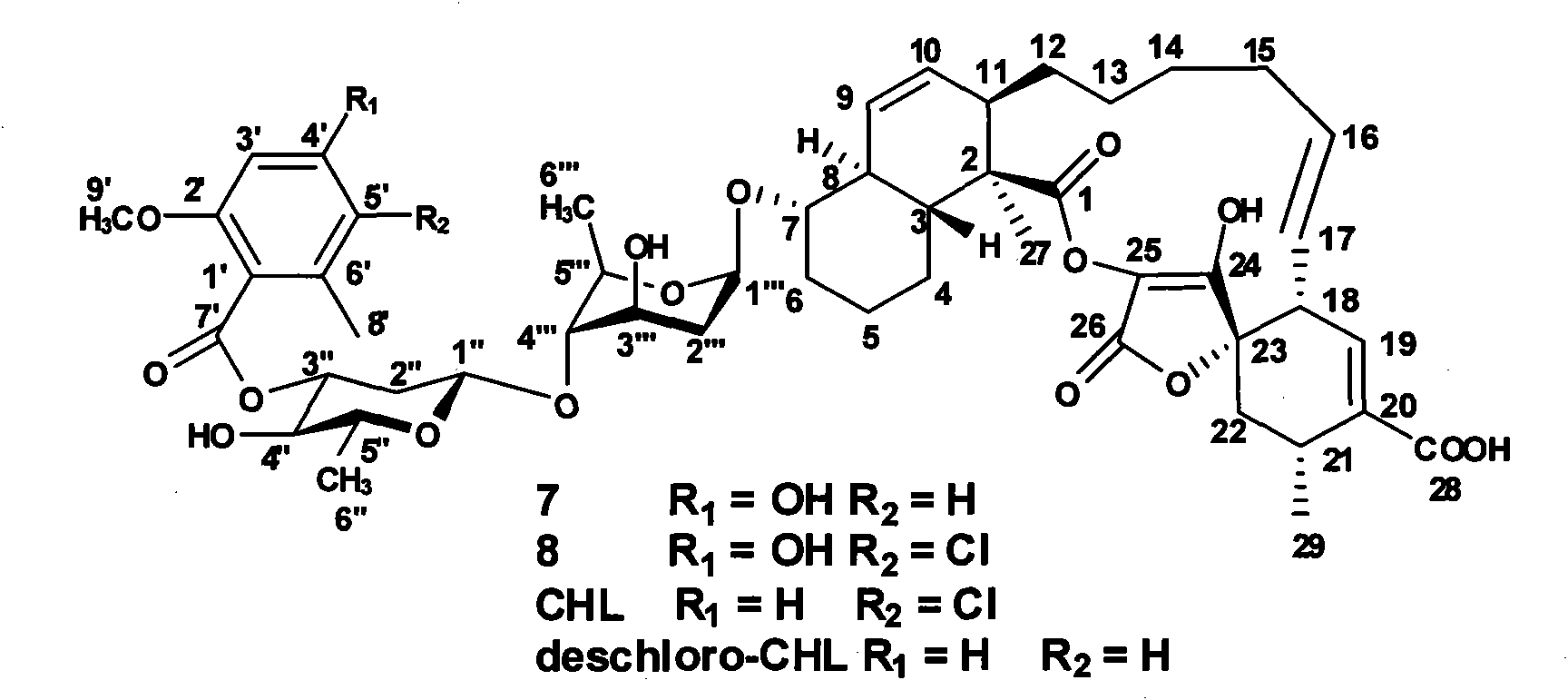
The invention discloses a novel method for synthesizing spirocyclic acetoacetic acid lactone antibiotic with higher activity by using combinatorial biosynthesis based on a genetic engineering method. The method is characterized by carrying out specific locus mutation on the keto-reduction (KR) conservation activity locus of a 6-methy salicylic acid (6-MSA) synthetase and genetically transforming the producing strain Streptomyces antibioticus DSM40725 of chlorothrichin (CHL) and fermenting to generate new chlorothrichin analogues 7 and 8 with improved biological activity. The favorable biological activity of the new chlorothrichin analogues 7 and 8 has values to be developed into medicaments.
Description
technical field The invention belongs to the field of biotechnological engineering, and specifically relates to the function of genetic engineering modification of 6-MSA synthetase, the fermentation, separation, structure identification and activity determination of new compound biosynthesis in recombinant bacterial strain combination. Background technique Natural compounds are a major source of drug discovery and development, such as penicillin and erythromycin for anti-infection, bleomycin and etomycin for anti-tumor, avermectin for anti-parasite and immunosuppressant cyclosporine etc., have been the main drugs for human treatment of diseases in the past few decades. Therefore, the research and development of new drugs based on natural compounds has always been a key area of concern in the chemical and medical circles. Among them, the natural products derived from microorganisms account for an important part. There are thousands of microbial natural products reported so...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H17/08C12N9/00C12N15/52C12N15/76C12P19/62A61K31/7048A61P31/04A61P35/00
Inventor 刘文丁伟
Owner 国科新研国际技术转移有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com