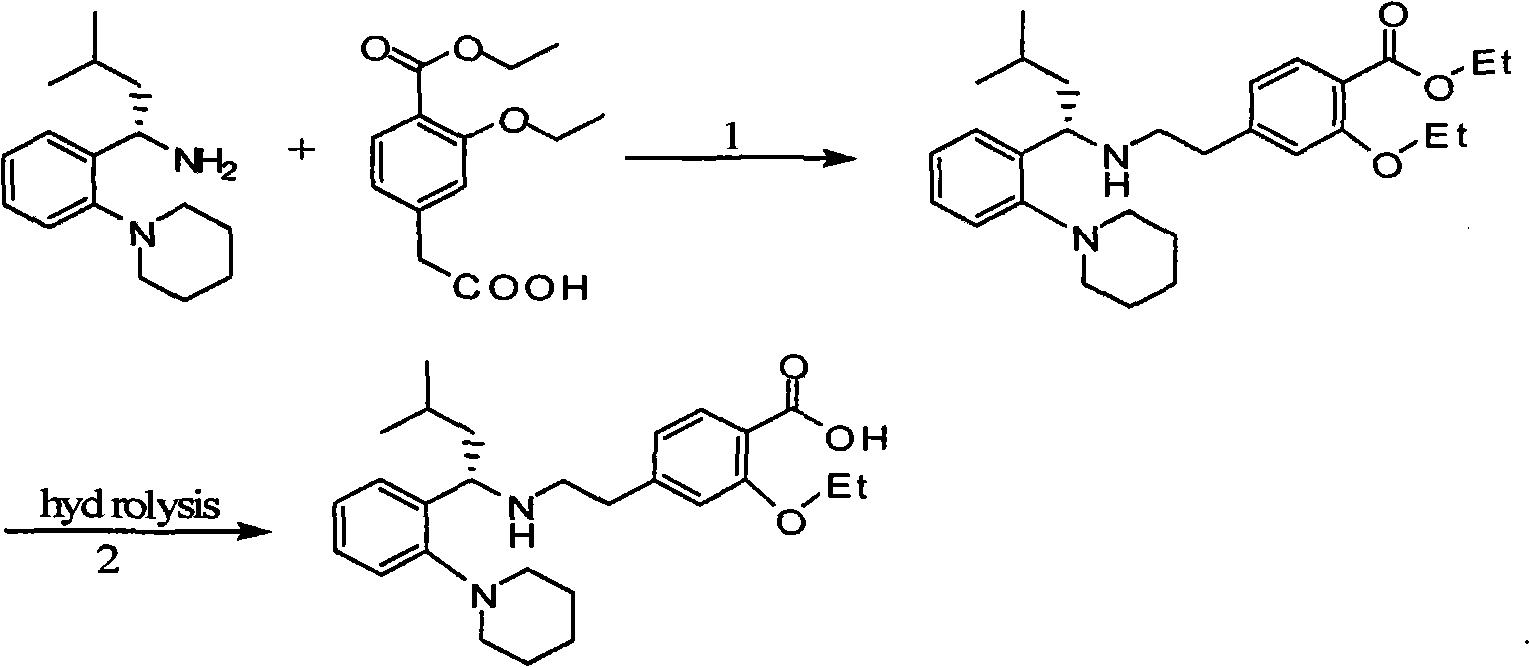
Preparation method of 3-ethyoxyl-4-carboxylphenylacetic acid
A technology of ethyl ethoxybenzoate and ethoxy, which is applied in the field of preparation of 3-ethoxy-4-carboxyphenylacetic acid, and can solve problems such as affecting the quality of the final product of repaglinide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
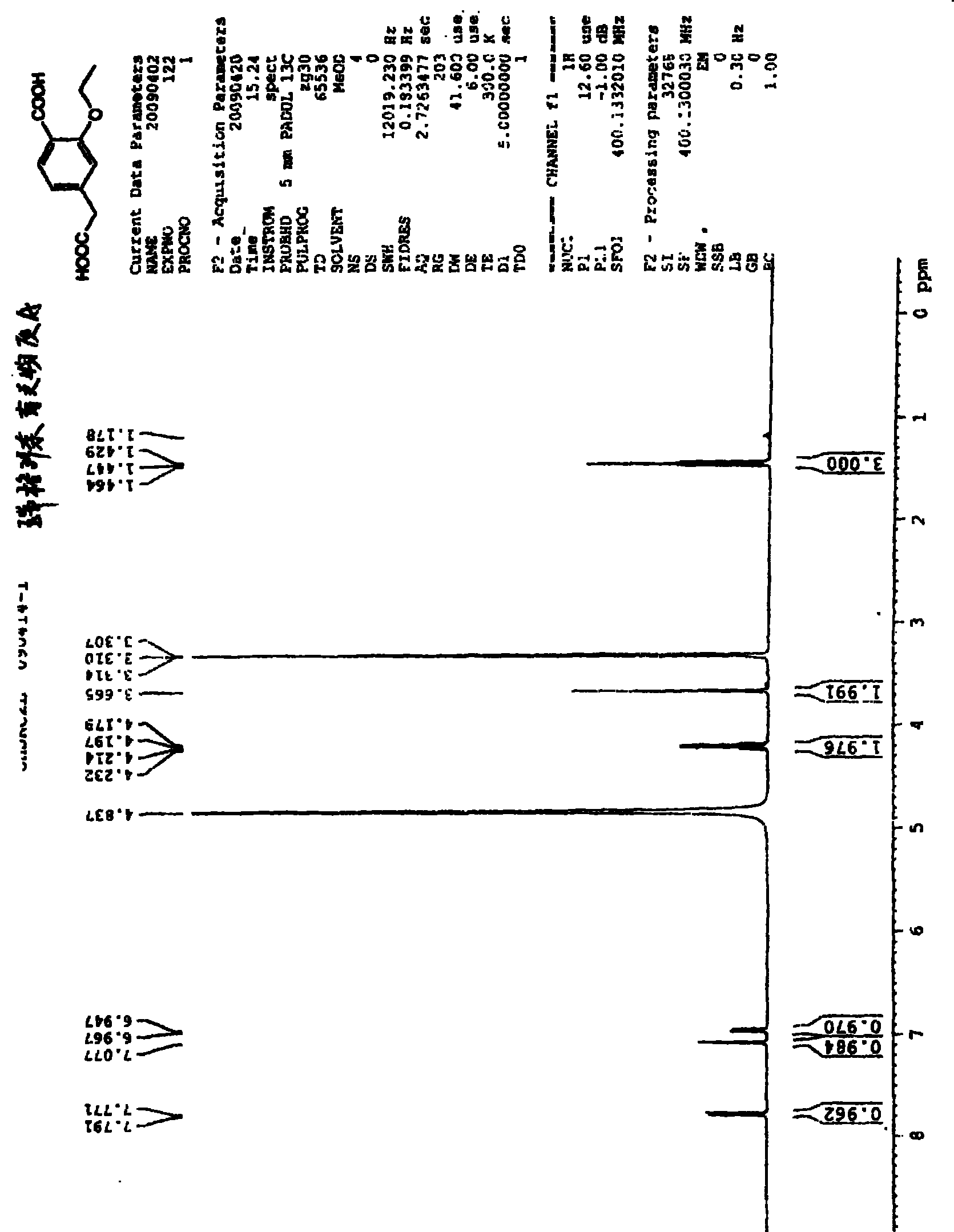
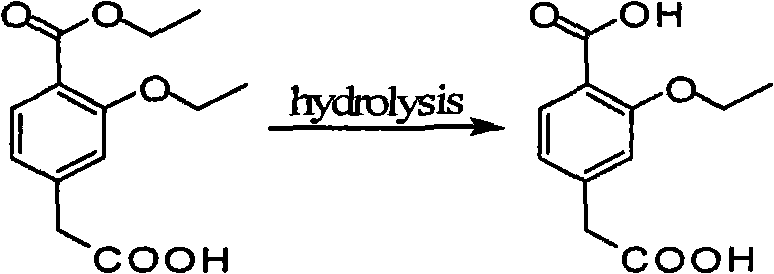
[0015] The preparation of example one 3-ethoxy group-4-carboxyphenylacetic acid
[0016] Take 5g of ethyl 4-carboxymethyl-3-ethoxybenzoate and add it to a 150ml three-neck flask with a condenser tube, add 50ml of ethanol to dissolve, heat up to 40°C, add 15.5ml of 1mol / L sodium hydroxide solution, Heat up to 60°C, keep stirring for 2h, cool down to 35°C, adjust pH to 5 with 1mol / L hydrochloric acid, keep stirring at 35°C for 30min, cool down to -5°C~0°C, stir for 1h, put in refrigerator for analysis Crystal overnight.
[0017] The above crystallization liquid was taken out, and colorless needle-like crystals were found to be precipitated. The filter cake was washed twice with 5 ml of water, and the obtained solid was vacuum-dried overnight at 50° C. to obtain 1.6 g of needle-like crystalline solid. Yield 36%, HPLC
[0018] Purity 96.83%.
example 2
[0019] The preparation of example two 3-ethoxy-4-carboxyphenylacetic acid
[0020] Take 5g of ethyl 4-carboxymethyl-3-ethoxybenzoate and add it to a 250ml three-neck flask with a condenser tube, add 100ml of ethanol to dissolve it, then heat up to 40°C, add 50ml of 1mol / L sodium hydroxide solution, and heat up to 60°C, control the temperature at 60°C ~ 65°C, keep stirring and react for 2 hours, apply the sample, after there is no raw material spot, cool down to 35°C, adjust the pH to 5 with 1mol / L hydrochloric acid, keep stirring at 35°C for 30min, Cool down to -5°C to 0°C, stir for 1 hour, and put it in the refrigerator for crystallization overnight.
[0021] The above crystallization liquid was taken out, and a large number of colorless needle crystals precipitated out. The filter cake was washed twice with 10 ml of water, and the obtained solid was vacuum-dried overnight at 50° C. to obtain 3.8 g of needle-like crystalline powder. The yield was 85.5%, and the purity by HPL...
example 3
[0022] The preparation of example three 3-ethoxy-4-carboxyphenylacetic acid
[0023] Take 5g of ethyl 4-carboxymethyl-3-ethoxybenzoate and add it to a 150ml three-neck flask with a condenser tube, add 50ml of ethanol to dissolve it, then raise the temperature to 40°C, add 25ml of 2mol / L sodium hydroxide solution, and heat up To 60°C, control the temperature at 60°C~65°C, keep stirring and react for 2 hours, apply the sample, after there is no raw material spot, cool down to 35°C, adjust the pH to 5 with 1mol / L hydrochloric acid, keep stirring at 35°C for 30min , cooled to -5°C to 0°C, stirred for 1 hour, and placed in a refrigerator for crystallization overnight.
[0024] The above crystallization liquid was taken out, and a large amount of colorless needle crystals were precipitated. The filter cake was washed twice with 10 ml of water, and the obtained solid was vacuum-dried overnight at 50° C. to obtain 4.1 g of needle-shaped crystalline powder. The yield was 92.3%, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com