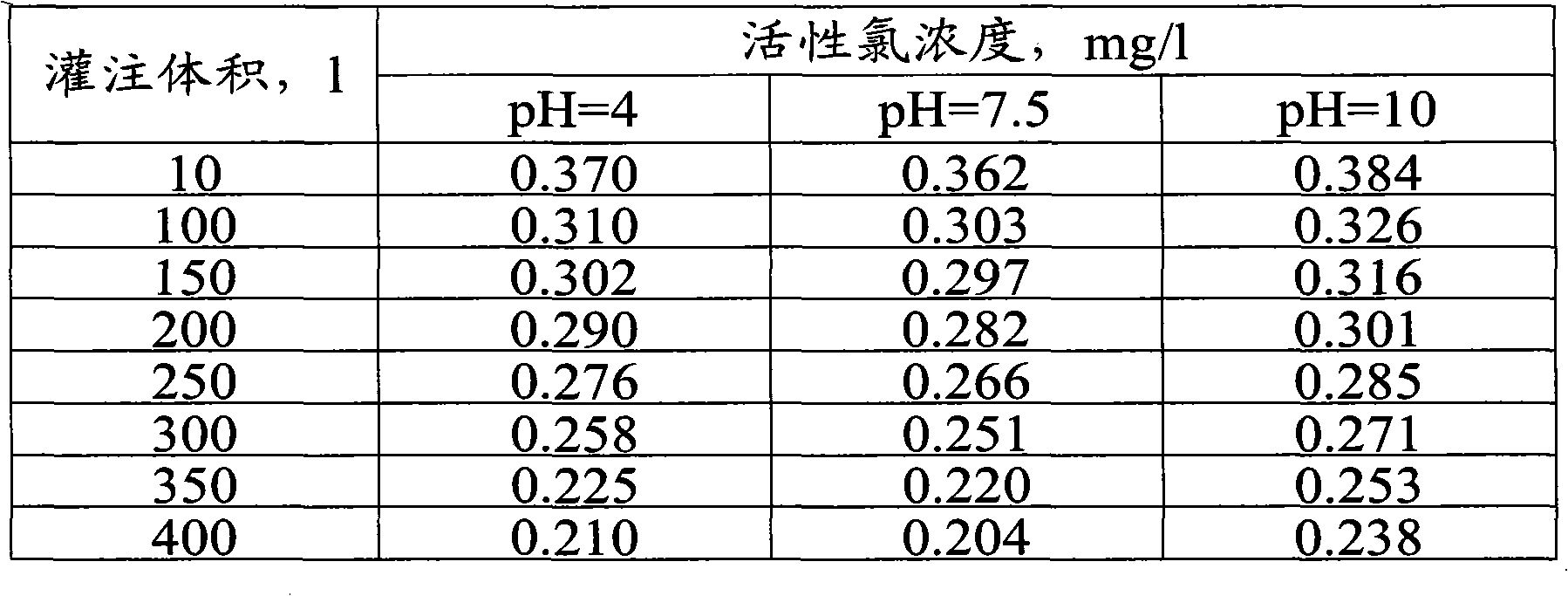
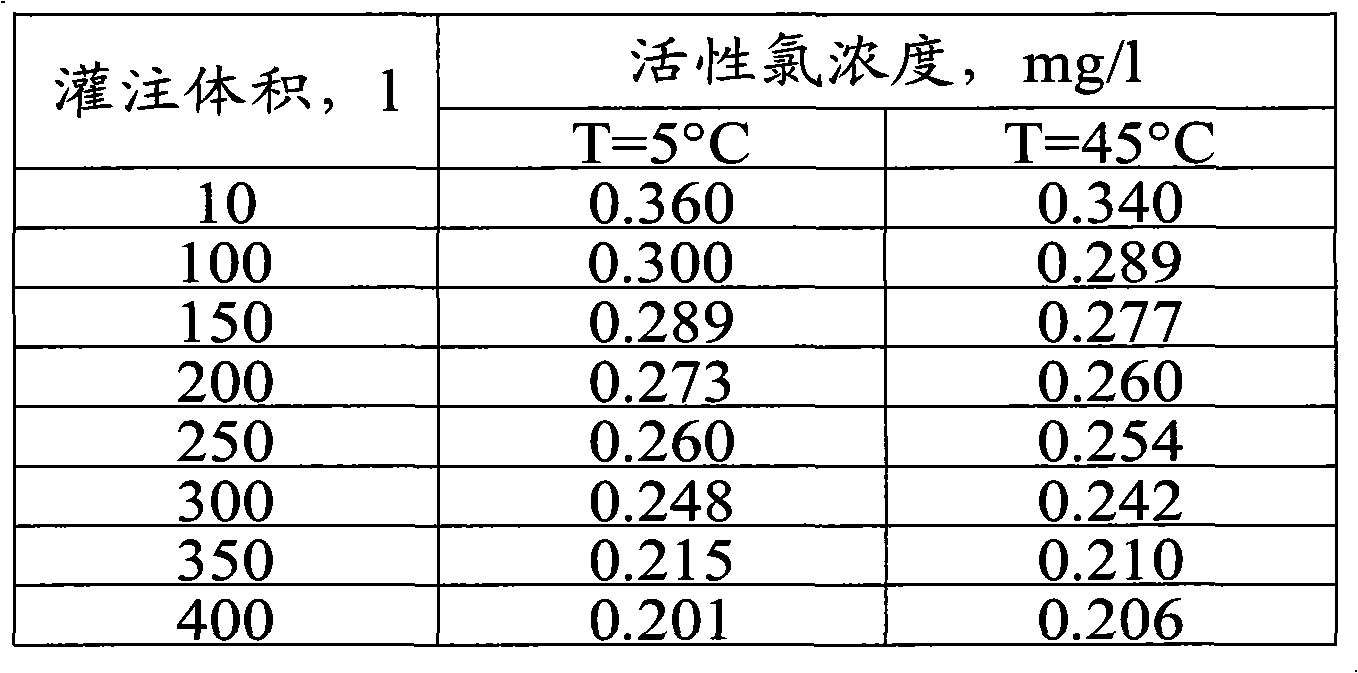
Antiseptic material and a method for the production and use thereof
A technology of anti-corrosion materials and polymer materials, applied in chemical instruments and methods, botanical equipment and methods, applications, etc., can solve the problems of complex power consumption and energy consumption, expensive, low ecological compatibility, etc., and achieve economical reduction and energy requirements, improved eco-compatibility, and the effect of increased eco-compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 22.7 grams (g) of melamine phosphate, 7.5 g of paraformaldehyde and 4 g of NaOH were placed in a round bottom flask (200 ml) equipped with a mechanical stirrer, followed by 100 ml of water. The mixture was vigorously stirred (1200 rpm) at 90°C for 4 hours. The product obtained is in the form of porous particles with a size ranging from 0.3 to 2 mm. The mass of the obtained product was 29.8 g, and the molar ratio of melamine derivative / phosphorus / formaldehyde derivative was 110 / 92 / 2.47. The mole fraction of terminal methylol groups in the formaldehyde derivative is 0.013 (according to infrared spectroscopic data).
[0027] Particulate matter was flushed with a Buchner funnel.
[0028] The obtained polymer was chlorinated with 300 ml of a sodium hypochlorite solution having an active chlorine concentration of 52 g / l for 1 hour while stirring.
[0029] The chlorinated product was rinsed with water using a Buchner funnel until neutral, then dried at 50°C.
[0030] The pro...
Embodiment 2
[0032] 12.6 g melamine, 7.5 g paraformaldehyde and 21.0 g sodium dihydrogen phosphate dihydrate were charged into a round bottom flask (200 ml) equipped with a mechanical stirrer, followed by 100 ml water. The mixture was vigorously stirred (1200 rpm) at 90°C for 4 hours. The product obtained is porous, in the form of granules with a size of 0.3-2 mm. The mass of product obtained was 29.2 g.
[0033] The molar ratios of the components are the same as in Example 1.
[0034] Particulate matter was washed with water using a Buchner funnel.
[0035] The obtained polymer was chlorinated with 300 ml of sodium hypochlorite solution having an active chlorine concentration of 52 g / L for 1 hour while being stirred.
[0036] The chlorinated product was rinsed with water using a Buchner funnel until neutral, then dried at 50°C.
[0037] The product contained 28% active chlorine (chlorine content estimated by iodometric titration).
Embodiment 3
[0039] The polymeric material was obtained by the method described in Example 1.
[0040] The product obtained was chlorinated while being mixed with an aqueous suspension of bleaching powder (80 g of CaCl(OCl)) and kept for 5 hours.
[0041] The chlorinated product was rinsed with acid (100 ml of a 10% solution) and water using a Buchner funnel and then dried at 50°C.
[0042] The product obtained contained 26% active chlorine.
[0043] Examples demonstrating the performance and intended use of anticorrosion materials:
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com