Peptide slow-release formulations
A pre-preparation and active agent technology, applied in the field of preparation precursors, can solve the problems of difficulty in preparing PLGA microbeads, inability to heat sterilize, inability to filter sterilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Availability of Multiple Liquid Crystal Phases in Depot Formulations for Choice of Composition
[0192] Injectable formulations containing various ratios of phosphatidylcholine ("PC" - Epikuron 200) and glyceryl dioleate (GDO) and ethanol as solvent were prepared to illustrate the accessibility of the depot precursor formulation after equilibration with excess water Various liquid crystal phases.
[0193] Weigh an appropriate amount of PC and ethanol in a glass vial, and place the mixture on a shaker until the PC is completely dissolved to form a clear liquid solution. GDO is then added to form an injectable homogeneous solution.
[0194] Each formulation was injected into vials and equilibrated with excess water. Phase properties were evaluated visually and between cross-polarizations at 25 °C. The results are listed in Table 1.
[0195] Table 1
[0196]
[0197] L 2 = reverse micellar phase; I 2 = anti-cubic liquid crystal phase; H II = inverse hexagonal li...
Embodiment 2
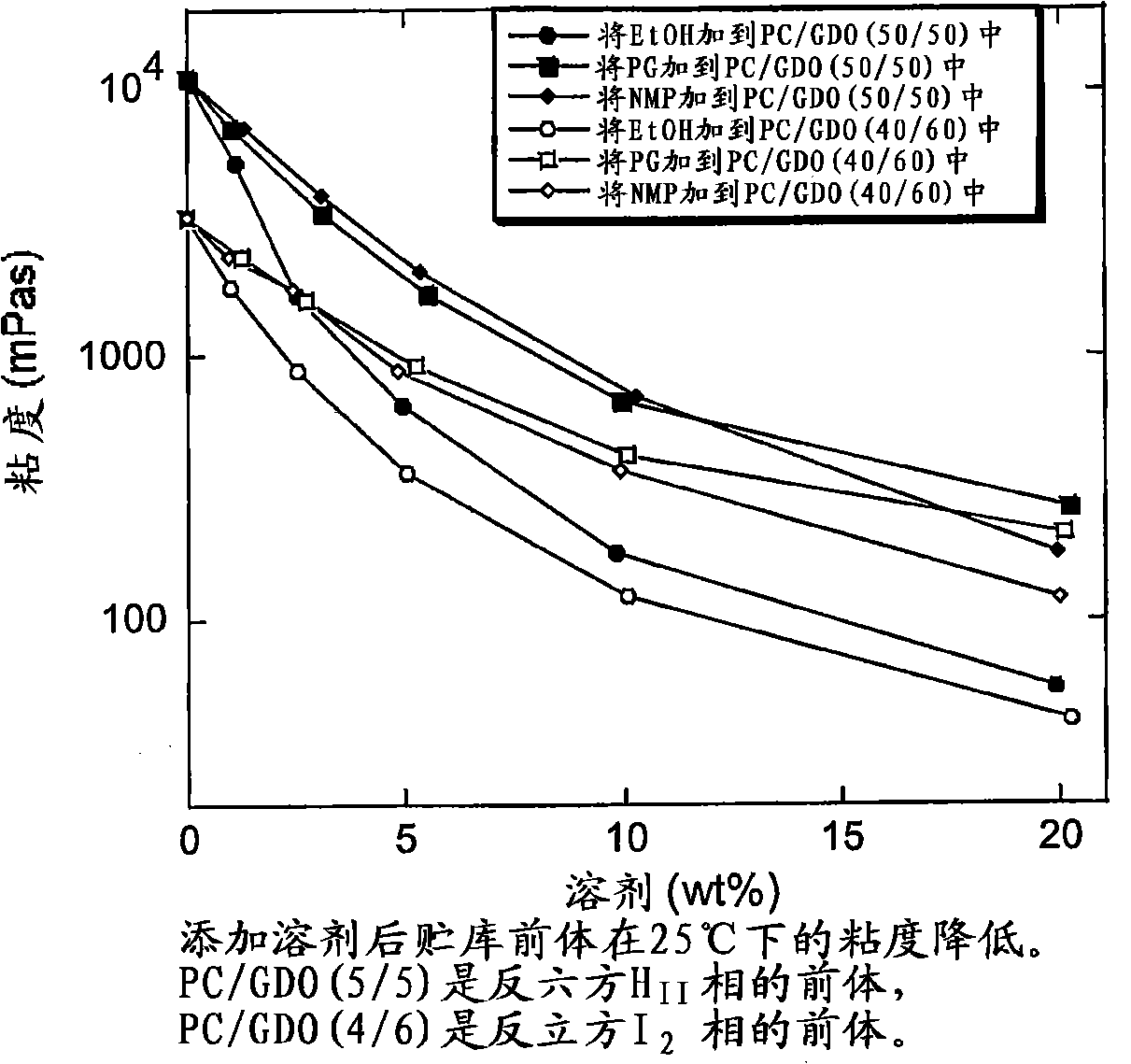
[0199] Viscosity in PC / GDO (5:5) or PC / GDO (4:6) with added solvents (ethanol, PG, and NMP)
[0200] A mixture of about 25% ethanol and PC / GDO / EtOH was prepared according to the method in Example 1. Use a rotary evaporator (vacuum, 1 hour at 40°C, then 2 hours at 50°C) to remove all or almost all of the ethanol in the mixture, weigh the resulting mixture in a glass vial, and then add 1, 3, 5, 10 Or 20% solvent (ethanol, propylene glycol (PG) or n-methylpyrrolidone (NMP)). The samples were allowed to equilibrate for several days, then the viscosity was measured with a CarriMed CSL 100 viscometer equipped with automatic notch setting.
[0201] This example clearly illustrates the need for solvents for some depot formulation precursors in order to obtain injectable formulations (see figure 1 ). The viscosity of solvent-free PC / GDO mixtures increases with increasing PC ratio. Systems with low PC / GDO ratios (more GDO) with low concentrations of solvent are injectable.
Embodiment 3
[0202] Example 3: Preparation of depot compositions containing the peptide octreotide
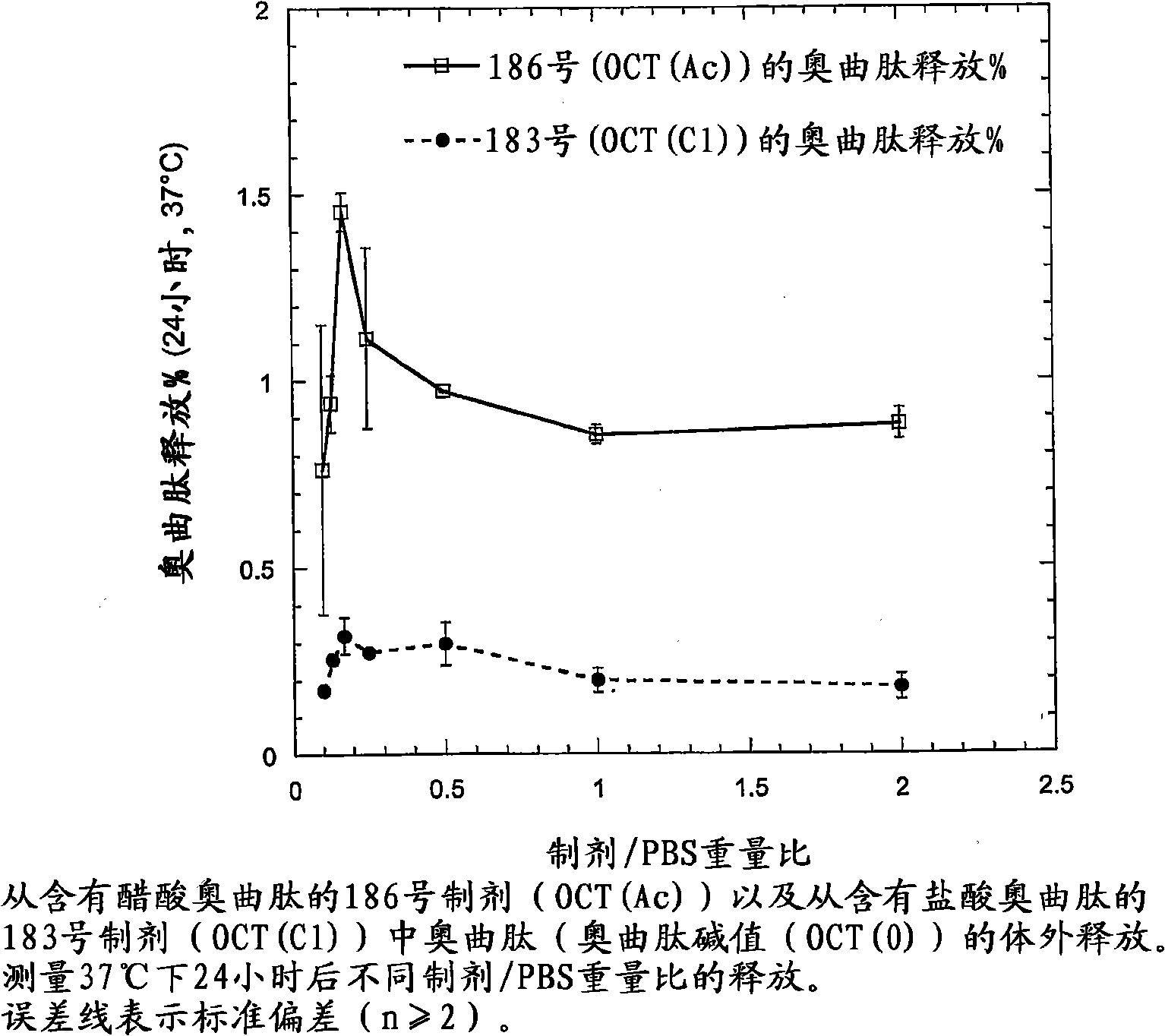
[0203] Octreotide acetate (24 mg or 60 mg) was dissolved in 0.1 g of ethanol. 0.36 g of PC and 0.54 g of GDO were sequentially dissolved in this solution to obtain a depot preparation precursor. The formulation precursor was injected into excess aqueous phase (syringe 23G, 0.6mm x 30mm) to obtain bulk liquid crystalline phase (I 2 structure). That is, octreotide (2.4% or 6.0%) did not change the overall composition and phase properties after exposure to an aqueous environment.
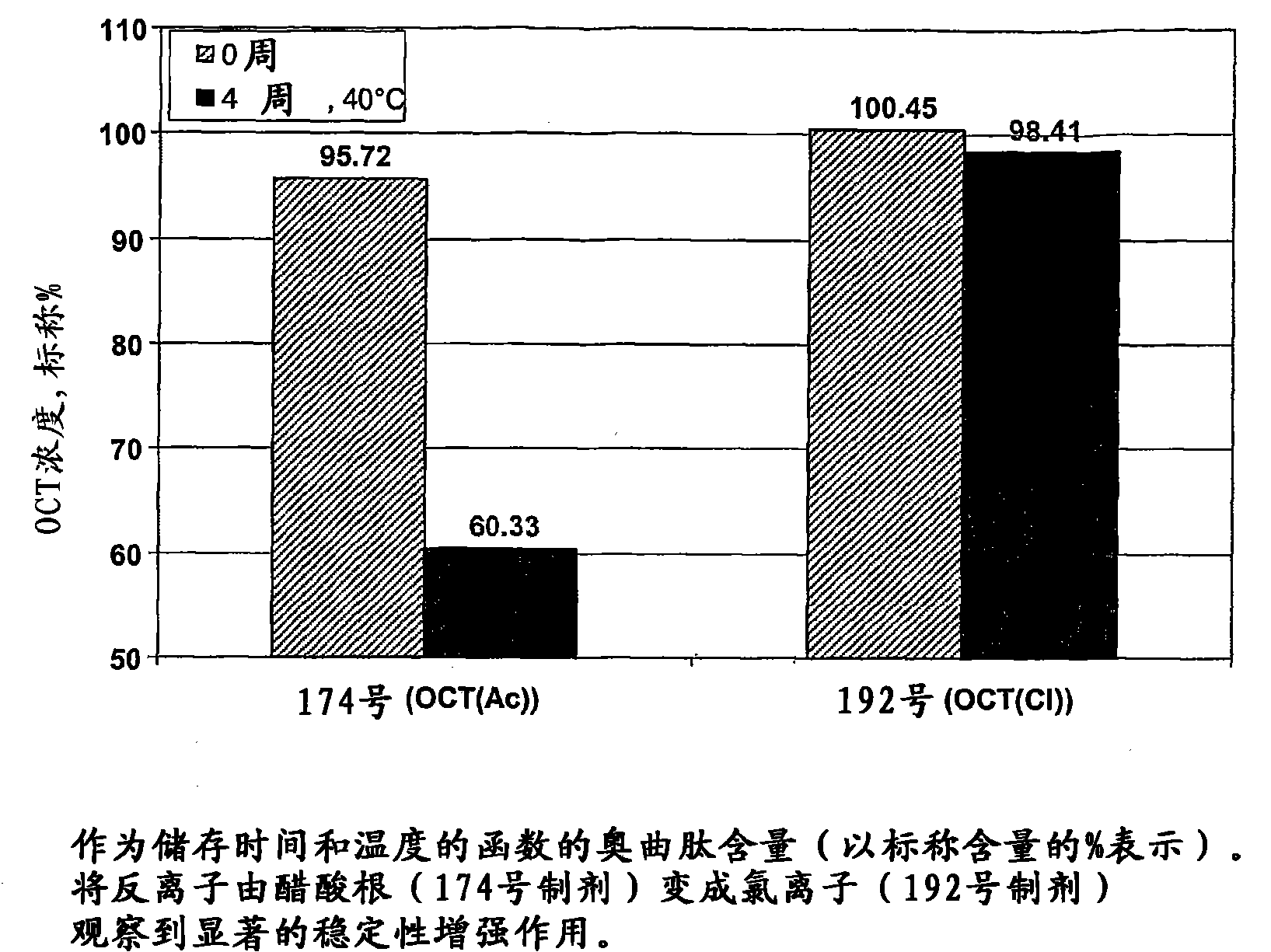
[0204] The octreotide depot precursor formulation in this example was tested for stability to crystallization during storage. Each formulation was stable for at least two weeks at 4-8°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com