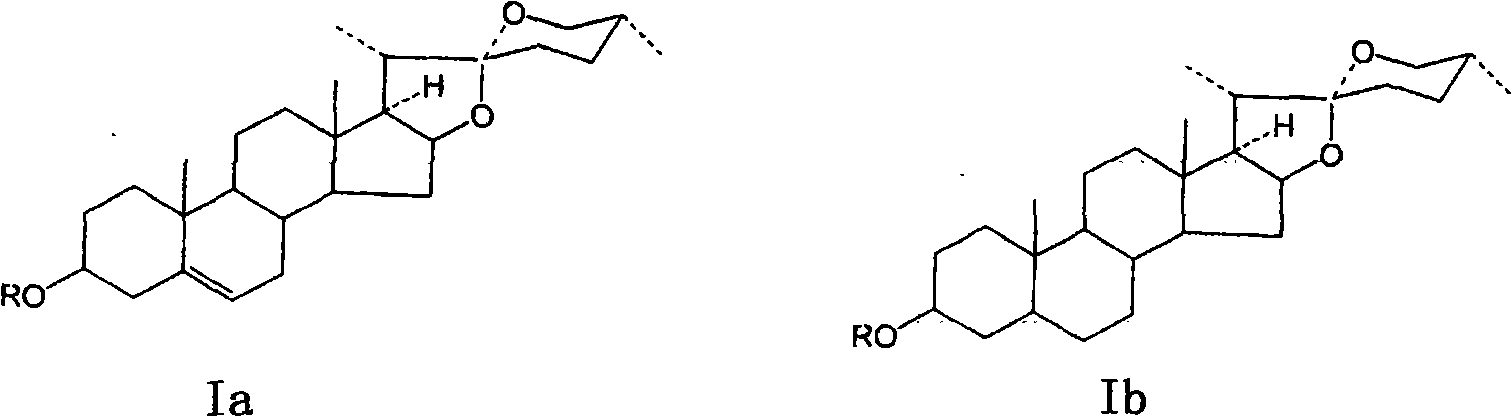
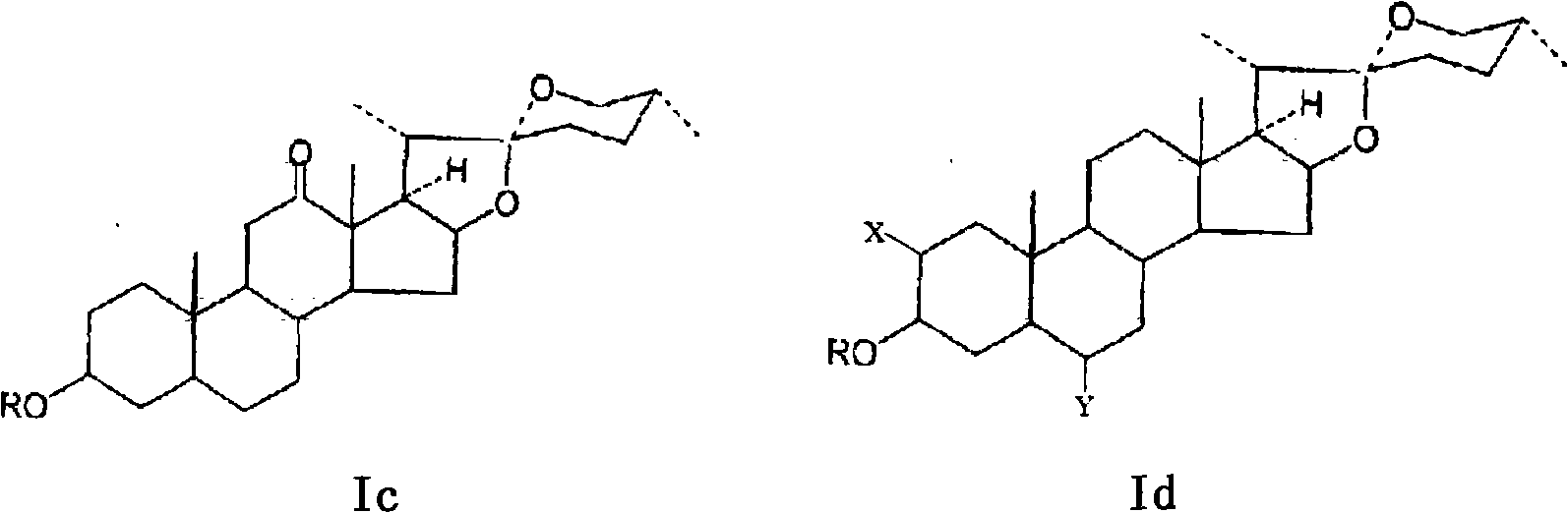
Method for preparing secondary steroidal saponin and diosgenin by catalyzing, oxidizing and degrading steroid general saponin
A technology of steroidal saponins and steroidal saponins, applied in chemical instruments and methods, preparation of steroids, organic chemistry, etc., can solve the problems of reducing production costs, environmental pollution, etc., reduce side reactions, and facilitate separation and purification , easy separation and recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Preparation method of steroidal total saponins
[0036] (1) Preparation of total saponins of Dioscorea: take Dioscorea decoction pieces as raw material, extract three times with 70%, 80% and 90% ethanol respectively after crushing, recycle ethanol after merging until a large amount of microcrystalline precipitates, and collect microcrystalline by filtration Solid (a); Add 95% ethanol to the filtrate until no precipitate separates out, filter to remove the precipitate, concentrate the filtrate under reduced pressure to dry solid (b), combine solids a and b to obtain thick dioscin; use 85% ethanol from crystallization Alternatively, it is eluted with water and 80% ethanol on a macroporous resin column, and the ethanol fraction is collected to obtain refined total saponins.
[0037] (2) Preparation of total saponins from stems and leaves of Chonglou: take the stems and leaves of the aerial part of Chonglou as raw material, extract with ethanol after crushing, reclaim the w...
Embodiment 1
[0040] The preparation method of embodiment 1 dioscin / pachyphylloside (Ia2)
[0041] (1) In a 1L autoclave, add 20g of dioscin total saponins, dissolve in 100mL of 85-90% ethanol, add 2g of active oxygen catalyst ENO100 or ENO200, fill in 0.2MPa oxygen under airtight conditions, and gradually heat up to 90-100°C, after 10 hours of reaction, lower the temperature and pressure to normal temperature and pressure, open the reactor and pour out the reaction solution, filter to remove the catalyst and a small amount of insoluble matter, and concentrate the filtrate until most of the ethanol is evaporated, and a large amount of white solid Precipitated, filtered, washed with water, dried, extracted with petroleum ether (60-80°C) to remove a small amount of saponin (Ia1), and the remaining solid was recrystallized with 95% ethanol to obtain 6.6-7.0g of the product , white microcrystalline solid Ia2 with a purity of 92% (HPLC).
[0042] (2) In a 1L autoclave, add 20g of total saponins...
Embodiment 2
[0043] The preparation method of embodiment 2 dioscin / pachyphylloside (Ia3)
[0044] (1) In a 1L autoclave, add 20g of dioscin total saponins, dissolve in 100mL of 85-90% ethanol, add 1g of active oxygen catalyst ENO100 or ENO200, fill in 0.1MPa oxygen under airtight conditions, and gradually heat up to 80-90°C, after 7 hours of reaction, lower the temperature and pressure to normal temperature and pressure, open the reactor and pour out the reaction solution, filter to remove the catalyst and a small amount of insoluble matter, and concentrate the filtrate until most of the ethanol is evaporated, and a large amount of white solid Precipitated, filtered and washed with water, dried, and then eluted with 70-85% ethanol on a silica gel column to obtain 1.4-1.6 g of Ia2 with a purity of 92% and 7.8-8 g of white microcrystalline with a purity of 93% (HPLC) Solid Ia3.
[0045] (2) In a 1L autoclave, add 20g of total saponins from the stems and leaves of Pachyphylla, dissolve in 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com